Scroll to:
Plasma Level’s of Neuregulin-1 in Healthy People
https://doi.org/10.20996/1819-6446-2021-11-01
Abstract
Aim. To determine the median levels of neuregulin-1 (NRG-1; endothelium-derived growth factor and the natural agonist of the ERBB3 and ERBB4 receptors) NRG-1 in healthy volunteers and to study the associations of NRG-1 levels with gender and age.
Material and Methods. Ninety seven healthy participants were enrolled (median age of 44 [32-54], men 45 men [46.4%]). The following age groups were identified: 20-29 y.о. (n=20, men – 50.0%), 30-39 y.о. (n=21, men – 52.4%), 40-49 y.о. (n=22, men – 45.5%), 50-59 y.о. (n=22, men – 36.4%); 60-69 y.о. (n=12, men – 50.0%). Peripheral blood samples were collected at the time of enrolment, standard laboratory tests were performed, and NRG-1 levels were determined in the plasma samples by ELISA.
Results. In the cohort of 97 healthy participants the median value of NRG-1 was 0.3 [0.121-2.24] ng/ml. NRG-1 levels did not differ significantly between men and women (p=0.145), indicating that NRG-1 levels are not influenced by gender. The levels of NRG-1 were similar in the different age groups: age 20-29 years=0.26 [0.17-0.37] ng/ml; age 30-39=0.24 [0.1-0.39] ng/ml; age 40-49=0.31 [0.19-1.15] ng/ml; age 50-59=0.37 [0.19-1.0] ng/ml; age 60-69=0.4 [0.13-0.81] ng/ml. Correlation analysis between NRG-1 levels and route blood measurements (haemoglobin, lipids, glucose, creatinine, and uretic acid) did not show significant associations.
Conclusions. In this study, the median value of NRG-1 plasma levels were determined. The results of the study show that age and gender had no influence on NRG-1 values.
For citations:
Zhbanov K.A., Shchendrygina A.A., Zheleznykh E.A., Privalova E.V., Suvorov A.Y., Ablyametova A.S., Fuksman N.F., Salakheeva E.Yu., Belenkov Yu.N. Plasma Level’s of Neuregulin-1 in Healthy People. Rational Pharmacotherapy in Cardiology. 2021;17(6):853-859. https://doi.org/10.20996/1819-6446-2021-11-01
Introduction
Neuregulin-1 (NRG-1) is an endothelial growth factor and a natural agonist of types 3 and 4 tyrosine kinase receptors (ERBB3, ERBB4) located on the membrane of cardiomyocytes, fibroblasts, coronary endothelium, and inflammatory cells [1][4]. The synthesis of NRG-1 in the myocardium occurs in response to ischemia, excessive adrenergic stimulation, and oxidative stress [5]. Binding of NRG-1 to ERBB4 receptors of cardiomyocyte triggers a cascade of adaptive intracellular reactions [6][7], contributing to an increase in the life span of cells under conditions of pathological influences [8-10]. It has been shown that activation of the NRG-1/ERBB4 system in heart failure (HF) is a cardioprotective mechanism [2][10- 18]. On the background of therapy with recombinant NRG-1, patients with HF and reducer left ventricular ejection fraction (HFrEF) noted a significant sustained improvement in systolic function and the development of its reverse remodeling [12][13].
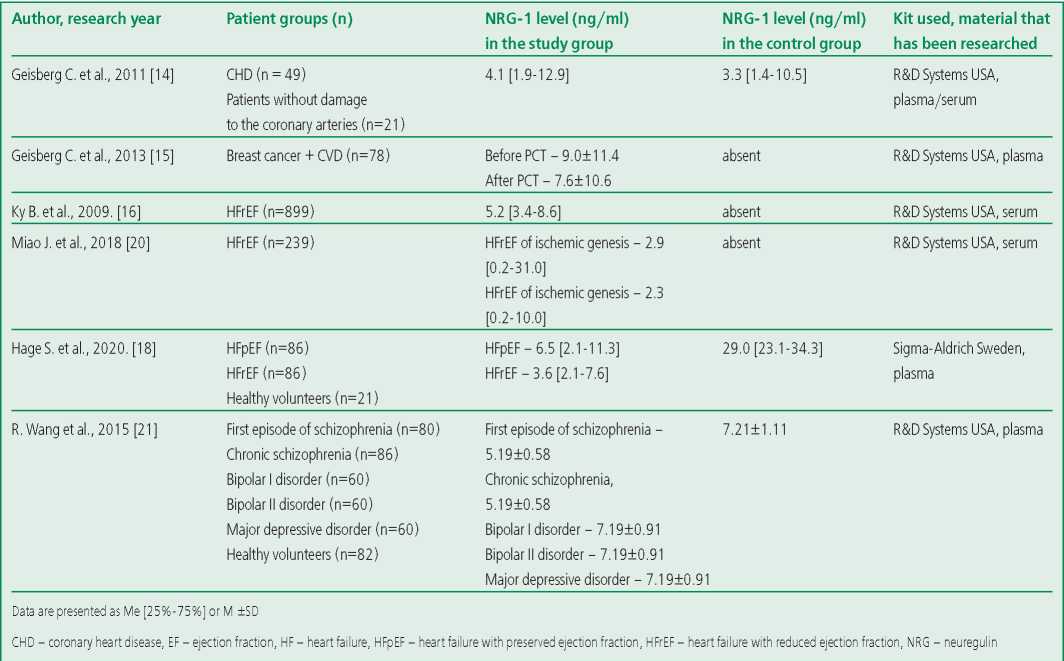
Earlier, it was repeatedly reported an increase in the NRG-1 level in serum/plasma of patients suffering from HF, ischemic heart disease [2][6][11-18][19][20]. Data on the NRG-1 level in healthy volunteers have not been studied in separate studies. Concentrations of this biomarker in healthy individuals who made up the control group in the above studies are contradictory (Table 1). The lack of data on the NRG-1 level in healthy individuals complicates the interpretation of changes in pathology. Thus, the determination of normal values of NRG-1 levels is necessary for further investigation of its role as a marker of the disease and a criterion for the effectiveness of the therapy with recombinant NRG-1. The aim of this study is to determine the concentration of circulating NRG-1 in the blood plasma of healthy people and to study the associations of NRG-1 levels with gender and age.
Table 1. NRG-1 levels in patients with cardiovascular diseases and healthy volunteers according to the results of previous studies
Materials and methods
97 healthy volunteers were included in the study. The majority of the participants were employees of the University Clinical Hospital No. 1 (Sechenov University). Inclusion criteria were age from 20 to 80 years, absence of diseases of the cardiovascular system, chronic diseases of the liver, kidneys, lungs, systemic inflammatory diseases and diabetes mellitus. All study participants underwent general and biochemical blood tests with determination of lipid spectrum parameters (total cholesterol [TC], low [LDL] and high density [HDL] lipoproteins by the colorimetric method using ADVIA® Chemistry Cholesterol Reagent, ADVIA® Chemistry Direct LDL Cholesterol Reagents, ADVIA® Chemistry Direct HDL Cholesterol Reagents), uric acid (colorimetrically using ADVIA® Chemistry Uric Acid Reagents), creatinine (by the kinetic method [Jaffe method] using ADVIA® Chemistry Creatinine Reagents), fasting glucose (by the hexokinase method using ADVIA® Chemistry Glucose Hexokinase II Reagents). The above parameters were investigated on a biochemical analyzer ADVIA® 2400 from Siemens Healthcare Diagnostics®. The indicators of a complete blood count, including hemoglobin, were determined using an XP 300 hematology analyzer from Sysmex®.
The NRG-1 level was determined in blood plasma samples that were previously frozen and stored at - 80°C. The concentration of NRG-1 was assessed by enzyme-linked immunosorbent assay using a Duoset ELISA kit (R&D Systems®, USA) to determine the level of the NRG-1b active peptide fragment in human blood plasma. The analysis results were recorded using a microplate reader (Luminometer Photometer LMA0B Beckman Coulter, 450 nm); data processing was performed using the 5PL algorithm. The concentration of NRG-1 in each sample was determined twice. The mean of the two values was used for analysis. The coefficient within the analysis series was 6.1%, between the analyzes it was 13.8%.
The study was carried out in accordance with the principles of the Declaration of Helsinki at the University Clinical Hospital No. 1 (Sechenov University).
Statistical analysis. Quantitative data are presented as median (Me) and interquartile range [ 25% -75% ], categorical data were described with absolute values and percentages. Comparison of percentages in the analysis of multifield contingency tables was performed using the Pearson's chi-square test. Comparison of the two groups in terms of a quantitative indicator, the distribution of which differed from normal, was performed using the MannWhitney's U-test. Comparison of three or more groups in terms of a quantitative indicator, the distribution of which was different from the normal, was performed using the Kruskal-Wallis's test, a posteriori comparisons were performed using Dunn's test with Holm's correction. Differences were considered statistically significant at p <0.05. The direction and closeness of the correlation between the two quantitative indicators were assessed using the Spearman's rank correlation coefficient (r [S]). Assessment of the effect of age, body mass index (BMI), parameters of general and biochemical blood analysis on the NRG-1 level was carried out during one-way regression analysis with the identification of the determination coefficient (R2). Statistical data processing was carried out using the statistical software package Statistica 10.0 (Statsoft Inc., USA).
Results
Characteristics of the examined patients
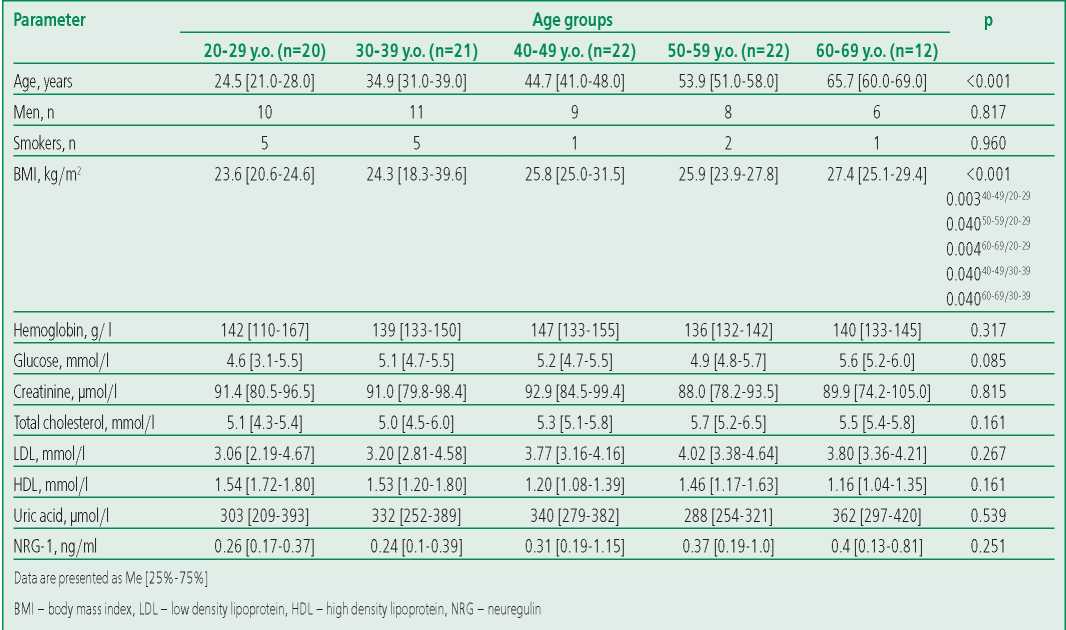
The study included 97 healthy volunteers, including 45 men (46.4%) and 52 women (53.6%) of the following age groups: 20-29 y.o. (n=20, men – 50.0%), 30-39 y.o. (n=21, men – 52.4%), 40-49 y.o. (n=22, men – 45.5%), 50-59 y.o. (n=22, men – 36.4%), 60-69 y.o. (n=12, men – 50.0%). The median age was 45 years [ 32-54]. Several participants had risk factors for cardiovascular disease. 19 people (19.5%) were smokers. Overweight (BMI=25-29.9 kg/m2) was noted in 34 (31.9%) participants, grade 1 obesity (BMI=30-34.9 kg/m2) was noted in 9 people (9.3%), obesity of the 2nd degree (BMI=35- 39.9 kg/m2) was noted in 4 (4.1%). The prevalence of abdominal obesity (waist circumference >80 cm in women and >94 cm in men) among women was 16.5% (n=16), and among men it was 4.1% (n=4). None of the included individuals had a family history of cardiovascular disease. The median hemoglobin level was 140 [ 133-153] g/l, glucose - 5.1 [ 4.6- 5.5] g/l, LDL – 3.56 [ 2.92-4.26] mmol/l, HDL – 1.16 [ 1.04-1.35] mmol/l, uric acid – 309 [ 252- 369] μmol/l. Detailed characteristics are shown in Table 2.
Table 2. Characteristics of the subjects
Plasma NRG-1 level in healthy volunteers, assessment of association with gender and age
The median NRG-1 level was 0.3 ng/ml [ 0.121- 2.24] in the general cohort of patients (n=97). There were no statistically significant differences between the concentrations of the studied biomarker in men and women (p=0.145; Fig. 1). NRG-1 levels were similar across age groups (Fig. 2). During univariate regression analysis, a significant but minimal effect of age on the NRG-1 level was noted (the determination coefficient [R2] was 0.03 at p=0.045) (Table 3).

Figure 1. NRG-1 serum level in men and women
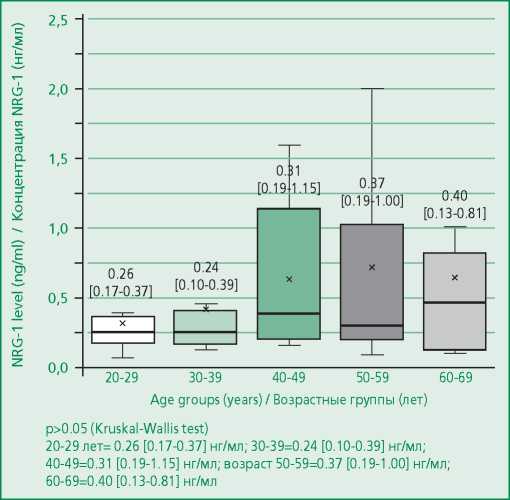
Figure 2. NRG-1 serum level in healthy volunteers depending on the age group
Assessment of the association of NRG-1 level with parameters of general and biochemical blood analysis
Correlation analysis didn't reveal a significant relationship between the NRG-1 concentration and the parameters of the general and biochemical blood analysis (Fig. 3): hemoglobin (r=-0.07, p=0.478), glucose (r=0.07, p=0.512), creatinine (r=-0.12, p=0.234), uric acid (r=-0.28, p=0.082). We also didn't reveal a statistically significant relationship with the parameters of the lipid spectrum: total cholesterol (r=0.14, p=0.254), LDL (r=0.20, p=0.174), HDL (r=0.001, p=0.994). Regression analysis didn't reveal the effect of laboratory parameters on the NRG-1 level (Table 3).
Table 3. Regression analysis of NRG-1 and basic laboratory parameters
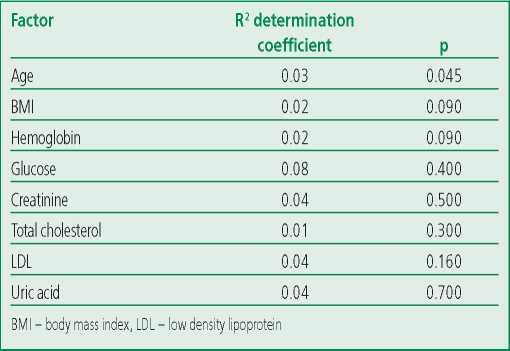
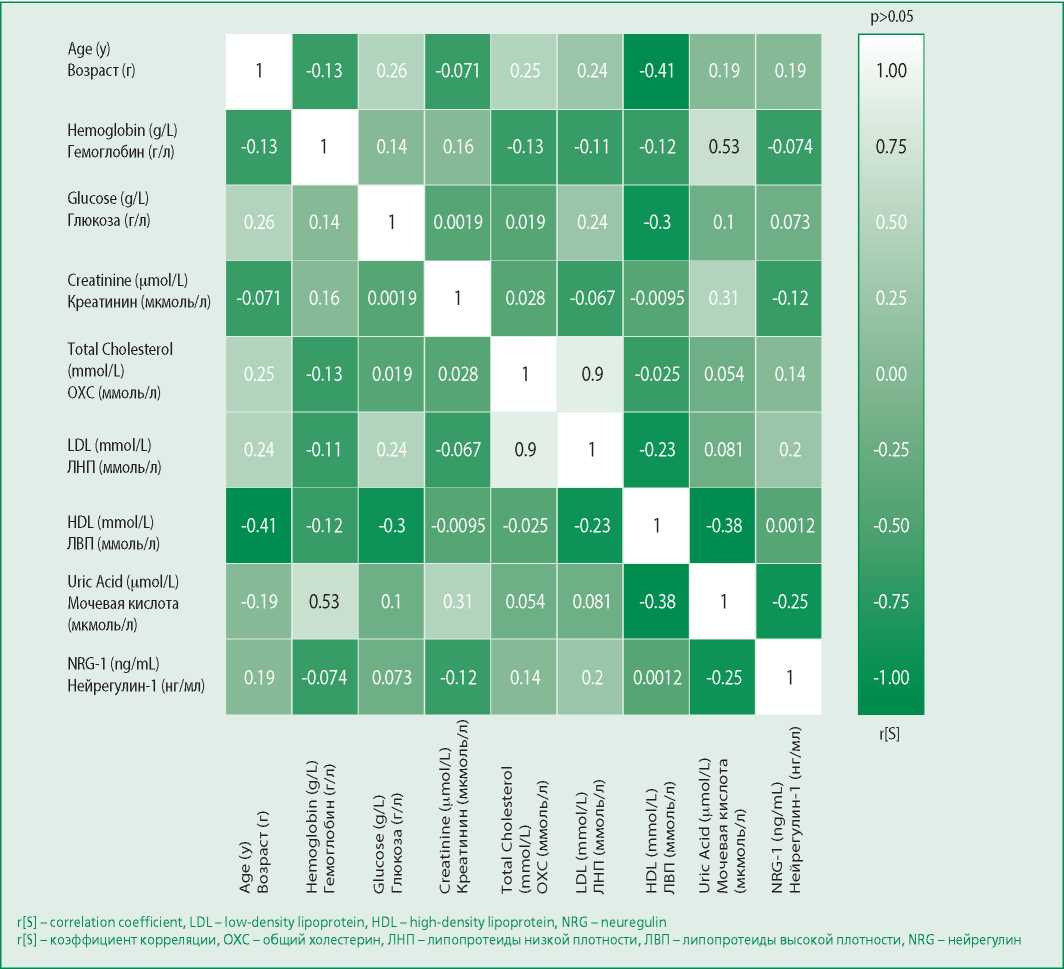
Figure 3. Matrices of rank correlation coefficients (r [S]) between NRG-1 levels and the studied parameters
Discussion
This study determined the plasma NRG-1 level in a relatively large cohort of healthy volunteers and showed the absence of an association of the studied biomarker with gender, age, parameters of general and biochemical blood analysis (hemoglobin, glucose, creatinine, uric acid), lipid spectrum.
Recently, a lot of attention has been paid to the study of the NRG-1 level in patients with various cardiac pathologies, but no separate studies have been carried out to determine the concentration of this biomarker in healthy individuals. The available data on the NRG-1 concentration in this category of individuals are contradictory (Table 1). For example, in the work of B. Ky et al, whose aim was to study the NRG-1 level in serum in patients with HFrEF, the median level of the biomarker was 5.2 [ 3.4-8.6] ng/ml [16]. In this study, there was no control group, data on the relationship of the biomarker with gender and age were not presented. C. Geisberg et al determined the NRG-1 concentration in patients with coronary heart disease [14]. The control group included patients who didn't have obstructive coronary heart disease. The NRG-1 level in this group was 3.3 [ 1.4-10.5] ng/ml [14], while the effect of gender and age on the biomarker concentration has not been studied either. C. Hage et al analyzed the NRG1 level in plasma in patients with HF and preserved ejection fraction (HFpEF) (N=86), HFrEF (n=86), and healthy volunteers (n=21) [18]. The NRG-1 levels in healthy individuals were quite high (29.0 [ 23.1-24.3] ng/ml) and significantly exceeded those in the HFrEF group – 3.6 [ 2.1-7.6] ng/ml; and in the HFpEF group – 6.5 [ 2.1-11.3] ng/ml. In addition, the resulting biomarker concentrations were significantly higher when compared to NRG-1 levels in other studies. Much attention is also paid to this marker by researchers studying the pathology of other body systems. For example, R. Wang's study determined the NRG-1 concentration in patients with schizophrenia, bipolar disorder, and depressive disorder, including a group of healthy volunteers (n=82, the average age was 29.5±5.8 years; 43 men), the NRG-1 concentration in the control group was 7.21±1.11 ng/ml, there were no statistically significant differences associated with gender (p=0.204). But we note that in fact one age group is represented [21]. Also, M. Shibuya et al found in a group of healthy volunteers that the NRG-1 concentration in men (n=20) - 5.61±0.91 ng/ml was significantly lower than in women (n=20) - 8.02±1.33 ng/ml (p=0.048), and, as in R. Wang's work, they represented one age group, the average age of which was 32.7±6.7 years [22].
Thus, the results of our study give an idea of the NRG-1 concentration in healthy individuals. The data obtained can be further used in studies to assess the clinical and prognostic significance of this biomarker in patients with various cardiac pathologies, in the study of the effectiveness of promising therapy in the treatment of CHF with recombinant NRG-1.
Limitations of this study: a relatively small sample of patients, as well as the use of an enzyme-linked immunosorbent assay kit from only one manufacturer – R&D Systems. NRG-1 level study using kits from other manufacturers (Sigma-Aldrich) is necessary.
Conclusion
In this study, we obtained data on the average NRG-1 level in healthy individuals, as well as the absence of an association of the studied biomarker with gender and age. The results of this work in the future can serve as a reference point for studies of changes in the NRG-1 level in individuals with various diseases, provided that a similar method for determining the biomarker is used.
Relationships and Activities. None.
Funding. The study was performed with the financial support of the Russian Foundation for Basic Research within the framework of scientific project №18-515-76002, scientific grant “The neuregulin1 pathway in development and progression cardiovascular disease: identification of small molecule ErbB4 agonists and identification of patient populations that could benefit the most”.
References
1. Ryzhov S, Matafonov A, Galindo CL, et al. ERBB signaling attenuates proinflammatory activation of nonclassical monocytes. Am J Physiol Heart Circ Physiol. 2017;312(5):H907-H918. DOI:10.1152/ajp-heart.00486.2016.
2. Liu W, Zscheppang K, Murray S, et al. The ErbB4 receptor in fetal rat lung fibroblasts and epithelial type II cells. Biochim Biophys Acta. 2007;1772(7):737-47. DOI:10.1016/j.bbadis.2007.04.008.
3. Xu Z, Ford GD, Croslan DJR, et al. Neuroprotection by neuregulin-1 following focal stroke is associated with the attenuation of ischemia-induced pro-inflammatory and stress gene expression. Neurobiol Dis. 2005;19(3):461-70. DOI:10.1016/j.nbd.2005.01.027.
4. Mograbi B, Rochet N, Imbert V, et al. Human monocytes express amphiregulin and heregulin growth factors upon activation. Eur Cytokine Netw. 1997;8(1):73-81.
5. De Keulenaer GW, Feyen E, Dugaucquier L, et al. Mechanisms of the Multitasking Endothelial Protein NRG-1 as a Compensatory Factor during Chronic Heart Failure. Circ Heart Fail. 2019;12(10):1-15. DOI:10.1161/CIRCHEARTFAILURE.119.006288.
6. Galindo CL, Ryzhov S, Sawyer DB. Neuregulin as a heart failure therapy and mediator of reverse remodeling. Curr Heart Fail Rep. 2014;11(1):40-9. DOI: 10.1007/s11897-013-0176-2.
7. García-Rivello H, Taranda J, Said M, et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289(3):H1153-60. DOI:10.1152/ajpheart.00048.2005.
8. Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol Heart Circ Physiol. 1999;277(6):H2205-11. DOI:10.1152/ajpheart.1999.277.6.h2205.
9. Lemmens K, Segers VFM, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006;281(28):19469-77. DOI:10.1074/jbc.M600399200.
10. Galindo CL, Kasasbeh E, Murphy A, et al. Anti‐Remodeling and Anti‐Fibrotic Effects of the Neuregulin‐1b Glial Growth Factor 2 in a Large Animal Model of Heart Failure. J Am Heart Assoc. 2014;3(5):000773. DOI:10.1161/jaha.113.000773.
11. Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 Signaling Induces Cardiomyocyte Proliferation and Repair of Heart Injury. Cell. 2009;138(2):257-70. DOI:10.1016/j.cell.2009.04.060.
12. Gao R, Zhang J, Cheng L, et al. A phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55(18):1907-14. DOI:10.1016/j.jacc.2009.12.
13. Jabbour A, Hayward CS, Keogh AM, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13(1):83–92. DOI:10.1016/j.jacc.2009.12.044.
14. Geisberg CA, Wang G, Safa RN, et al. Circulating neuregulin-1b levels vary according to the angiographic severity of coronary artery disease and ischemia. Coron Artery Dis. 2011;22(8):577-82. DOI:10.1097/MCA.0b013e32834d3346.
15. Geisberg CA, Abdallah WM, Da Silva M, et al. Circulating neuregulin during the transition from stage a to stage B/C heart failure in a breast cancer cohort. J Card Fail. 2013;19(1):10-5. DOI:10.1016/j.cardfail.2012.11.006.
16. Ky B, Kimmel SE, Safa RN, et al. Neuregulin-1b is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120(4):310-7. DOI:10.1161/CIRCULATIONAHA.109.856310.
17. Zeng Z, Gui C, Nong Q, et al. Serum neuregulin-1b levels are positively correlated with VEGF and Angiopoietin-1 levels in patients with diabetes and unstable angina pectoris. Int J Cardiol. 2013;168(3):3077-9. DOI:10.1016/j.ijcard.2013.04.088.
18. Hage C, Wärdell E, Linde C, et al. Circulating neuregulin1-b in heart failure with preserved and reduced left ventricular ejection fraction. ESC Hear Fail. 2020;7(2):445-55. DOI:10.1002/ehf2.12615.
19. Naresh A, Long W, Vidal GA, et al. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66(12):6412-20. DOI:10.1158/0008-5472.CAN-05-2368.
20. Miao J, Huang S, Su YR, Lenneman CA, et al. Effects of endogenous serum neuregulin-1b on morbidity and mortality in patients with heart failure and left ventricular systolic dysfunction. Biomarkers. 2018;23(7):704-8. DOI:10.1080/1354750X.2018.1485054.
21. Wang R, Wang Y, Hu R, et al. Decreased plasma levels of neureglin-1 in drug naïve patients and chronic patients with schizophrenia. Neurosci Lett. 2015;606:220-4. DOI:10.1016/j.neulet.2015.09.010.
22. Shibuya M, Komi E, Wang R, et al. Measurement and comparison of serum neuregulin 1 immunoreactivity in control subjects and patients with schizophrenia: An influence of its genetic polymorphism. J Neural Transm. 2010;117(7):887-95. DOI:10.1007/s00702-010-0418-3.
About the Authors
K. A. ZhbanovRussian Federation
Konstantin A. Zhbanov.
Moscow.
A. A. Shchendrygina
Russian Federation
Anastasia A. Shchendrygina.
Moscow.
E. A. Zheleznykh
Russian Federation
Elena A. Zheleznykh.
Moscow.
E. V. Privalova
Russian Federation
Elena V. Privalova.
Moscow.
A. Y. Suvorov
Russian Federation
Alexander Y. Suvorov.
Moscow.
A. S. Ablyametova
Russian Federation
Avasherfe S. Ablyametova.
Moscow.
N. F. Fuksman
Russian Federation
Nelli F. Fuksman.
Moscow.
E. Yu. Salakheeva
Russian Federation
Ekaterina Yu. Salakheeva.
Moscow.
Yu. N. Belenkov
Russian Federation
Yuri N. Belenkov.
Moscow.
Review
For citations:
Zhbanov K.A., Shchendrygina A.A., Zheleznykh E.A., Privalova E.V., Suvorov A.Y., Ablyametova A.S., Fuksman N.F., Salakheeva E.Yu., Belenkov Yu.N. Plasma Level’s of Neuregulin-1 in Healthy People. Rational Pharmacotherapy in Cardiology. 2021;17(6):853-859. https://doi.org/10.20996/1819-6446-2021-11-01
















































