Scroll to:
Sequelae of COVID-19 at long-term follow-up after hospitalization
https://doi.org/10.20996/1819-6446-2022-04-03
Abstract
Aim. To assess long-term sequelae of COVID-19 in hospitalized patients at 3 to 7 months after discharge.
Material and Methods. The whole of 700 patients hospitalized to the temporary COVID-19 treatment center hosted by the FSBI “National Medical Research Center of Cardiology” of the Ministry of Health of Russia from April to June 2020 were invited to participate in a follow-up study. At 3-7 months after the index hospitalization, patients or their proxies were contacted via telephone in order to obtain information on their vital status, cardiovascular and other conditions or their complications, and new hospitalizations. In addition, patients were invited to an outpatient visit under the "COVID-19-follow-up" program, encompassing physical examination and a comprehensive battery of laboratory and instrumental tests, including spirometry, chest computed tomography (CT) and the six minute walk test (6MWT). Further, dyspnea was assessed using the mMRC (Modified Medical Research Council) Dyspnea Scale.
Results: We were able to contact 87.4% (612/700) of patients or their proxies. At follow-up, 4.4% (27) patients died, of which 96.3% (26) had cardiovascular diseases (CVD). A total of 213 patients aged 19 to 94 years old (mean age 56.8±12.5, median 57 years [49.0; 64.0]; men, 55.4%) agreed come for an outpatient visit and to participate in the “COVID-19-follow-up” program. Since discharge, 8% (17) of patients required new hospitalizations, and more than a half of these patients (58.8%; 10/17) had CVD-related hospitalizations. A total of 8.4% (18) patients experienced worsening of hypertension, 9 (4.2%) patients had newly diagnosed hypertension, 2 (0.9%) – coronary artery disease patients experienced new/recurrent angina symptoms. 4 (1.9%) patients had newly diagnosed coronary artery disease, and one patient had an ischemic stroke. At the outpatient visit, 114 (53.5%) patients had some symptoms, most frequently, shortness of breath (33%), fatigue (27.4%), chest pain (11.3%), and abnormal heartbeats (8.5%). Based on the mMRC Scale, 59% of patients had dyspnea of varying severity. Most patients had a normal vital capacity (VC), which was moderately reduced in 3.3% and severely reduced in 0.5% of patients. Chest CT scans were obtained in 78 (36.6%) patients, whose worst lung damage scores during hospitalization were CT3 or CT4. One in ten patients (10.8%) with severe lung damage during acute infection had persisting ground glass opacities, 35.9% developed fibrotic changes, 79.6% of patients had linear or fine focal opacities. According to the 6MWT data, 12.3% of patients walked less than 70% of the predicted distance, 67% walked 71 to 99% of the predicted distance, and 20.7% of patients were able to walk 100% of their predicted distance.
Conclusion. These data suggest long-term negative sequelae of COVID-19 in more than half of hospitalized patients.
For citations:
Pogosova N.V., Paleev F.N., Ausheva A.K., Kuchiev D.T., Gaman S.A., Veselova T.N., Belkind M.B., Sokolova O.Yu., Zhetisheva R.A., Ternovoy S.K., Boytsov S.A. Sequelae of COVID-19 at long-term follow-up after hospitalization. Rational Pharmacotherapy in Cardiology. 2022;18(2):118-126. https://doi.org/10.20996/1819-6446-2022-04-03
Introduction
Since 2020, the spread of a new coronavirus infection (COVID-19) has become a pandemic [1]. This infectious disease, which is characterized by high contagiousness, has been recorded to date in more than 248 million people worldwide and has caused the death of more than 5 million patients [2]. COVID-19 primarily affects the respiratory system at the initial stages, but accumulated clinical data indicate the multisystem nature of the disease, which is often severe and can lead to death [3][4]. The severity of the disease steadily correlates with the age of patients and the presence of concomitant pathology. COVID-19 in hospitalized patients is often associated with cardiovascular diseases (CVD), in particular, with hypertension (AH), coronary artery disease (CHD), atrial fibrillation (AF), type 2 diabetes mellitus (DM) [5][6]. The listed diseases are significant unfavorable predictors of a more severe course of COVID-19, the development of complications and deaths [7][8].
The consequences of COVID-19 during long-term follow-up are not yet well understood, but there is growing evidence of various somatic consequences of the disease, cognitive impairment, reduced quality of life, and psycho-emotional disorders. Analysis of data from recent outbreaks of other coronavirus infections (acute respiratory distress syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) in 2012) showed that a quarter of patients who survived 6 months after hospitalization noted a decrease in lung function and exercise tolerance (according to a meta-analysis of 28 studies) [9].
Obviously, the study of the consequences of COVID-19 during long-term follow-up is an extremely urgent task today, which is of great medical and social importance.
The study aim is to assess the consequences of COVID-19 in hospitalized patients at 3 to 7 months after discharge.
Material and Methods
Patients who were hospitalized at the COVID treatment center of the FSBI «National Medical Research Center of Cardiology» of the Ministry of Health of Russia from April to June 2020 were invited to participate in the study 3-7 months after the index hospitalization. During the telephone contact, registration of vital status, cardiovascular and other diseases and their complications, as well as hospitalizations for the past period was carried out. All patients were offered to undergo a comprehensive outpatient examination at the FSBI «National Medical Research Center of Cardiology» of the Ministry of Health of Russia under the program «COVID-19 - long-term follow-up». The program included: examination by a doctor; recording complaints and smoking status; determination of body mass index (BMI), waist circumference and neck circumference; assessment of the dyspnea severity on the mMRC scale (Modified Medical Research Council); measurement of blood pressure (BP) and heart rate (HR); instrumental examination, including spirometry, chest multislice computed tomography (MSCT) (with an initial severity of lung damage 3-4); six-minute walk test (6MWT).
A comparative assessment was made of the frequency of detection of changes on chest CT and the degree of lung damage in the acute phase of the disease and at a long-term stage. The criteria for assessing the degree of lung damage were assessed by the severity index (SI), expressed in points [10]:
(а) «ground glass» was assessed visually according to the CT-index scale: from 0 to 5 points, depending on the amount of damage to the lung tissue in each lobe, where 0 means no damage; 1 - up to 5%; 2 – 6-25%; 3 – 26-50%; 4 – 51-75%; 5 – more than 75%; total: 0-25 points;
(b) consolidation was assessed by presence and number, where 0 means no cases; 1 – isolated cases; 2 – moderate; 3 – multiple consolidation zones; total: 0-15 points;
(c) indurations were assessed by presence and number, where 0 means no cases; 1 – isolated cases; 2 – moderate; 3 – multiple; total: 0-15 points;
(d) reticular changes were assessed by presence and number, where 0 means no cases; 1 – isolated cases; 2 – moderate; 3 – multiple; total 0-15 points;
(e) effusion was assessed by presence and severity, where 0 means no cases; 1 – insignificant; 2 – moderate; 3 – pronounced; total: 0-6 points;
(f) fibrosis was assessed by severity and prevalence in each lobe: 0 means no cases; 1 – isolated cases; 2 – moderate; 3 – multiple; total: 0-15 points.
The severity of each symptom was assessed separately for the right and left lungs, and then all points were summed up.
The assessment of the dyspnea severity, recommended by the Russian Ministry of Health for assessing the condition of patients after a new coronavirus infection [12], was carried out using the mMRC scale [11]. On the mMRC scale, the level of dyspnea corresponding to the patient's feelings was assessed from 0 to 4 points, where 0 means that dyspnea doesn't bother them, except for very intense exercise, and 4 points means extremely severe dyspnea that doesn't allow them to leave the house, or it appears when dressing and undressing.
Assessment of the level of physical performance was carried out using 6MWT [13]. During the 6MWT, patients had to walk as far as possible in 6 minutes at a pace acceptable to the patient (slowing down and/or stopping during the test was allowed, and the stopwatch was not stopped). After 6 minutes, we determined the number of meters passed by the patients. Before and at the end of the test, we assessed dyspnea and fatigue on the Borg's scale, determined heart rate and arterial oxygen saturation (SpO2). After the completion of 6MWT, we noted the possible appearance of symptoms. The distance traveled with 6MWT varies depending on gender, age, anthropometric parameters and other factors, but on average it ranges from 400 to 700 m in healthy people. We used the E.P. Enright and D. Sherrill's formula to calculate the predicted distance of 6MWT [14][15], according to which for men, the predicted distance of 6MWT (m) = (7.57×height in cm) - (5.02×age) - (1.76×weight in kg) - 309 m; for women, the predicted distance of 6MWT (m) = (2.11×height in cm) - (2.29×weight in kg) - (5.78×age) + 667 m.
We assessed the risk of cardiovascular complications using the SCORE scale or the 10-year global assessment of cardiovascular risk in the presence of atherosclerotic CVD, in accordance with the guidances in force at that time [16].
Statistical data analysis was performed using IBM® SPSS® Statistics version 23.0 (SPSS Inc., USA). Quantitative variables are presented as median (Me) and interquartile range [ 25%; 75%] or mean (M) and standard deviation (SD). For clarity, some qualitative ordinal variables (with the same median values) are presented simultaneously as Me [ 25%; 75%] and M±SD. We assessed the dynamics of 6MWT and MSCT indicators of the lungs using the Wilcoxon's test. Differences were considered statistically significant at a two-tailed p<0.05.
Results
A total of 700 patients were discharged from the COVID treatment center of the FSBI «National Medical Research Center of Cardiology» of the Ministry of Health of Russia after inpatient treatment from April to June 2020. 3-7 months after discharge, repeated attempts to establish telephone and mail contact with all discharged patients and/or their relatives were carried out. We were unable to establish contact with 88 patients (40 of them were residents of other regions). Contact was established with 612 (87.4%) patients.
27 (4.4%) patients (average age was 72.8±14.3 years) died within 3-7 months after discharge from the COVID center, of which 26 (96.3%) had CVD. 372 patients refused a face-to-face visit to the FSBI «National Medical Research Center of Cardiology», with the most common reasons for refusal being measures to reduce the spread of COVID-19 implemented by the Moscow government for people over 65 years of age and the fear of younger patients regarding re-infection in a medical institution.
As a result, 213 patients aged 19 to 94 years old (median age was 57 [ 49.0; 64.0] years) were included in the program «COVID-19 – term followup». The absolute majority of patients belonged to the age category of 40 years and older (Table 1).
Table 1. Essential clinical and demographic characteristics of patients included in the study (n=213)

The gender distribution was approximately equal with a slight male predominance.
The body mass index (BMI) averaged 29.4 kg/m2, while overweight or obesity (in most cases I-II degree) was observed in a significant part of patients. Severe abdominal obesity (waist circumference >102 cm in men and >88 cm in women) was detected predominantly in women (75 out of 138).
At the time of the examination, 7.1% of patients continued to smoke, 25.9% were ex-smokers, the rest of the patients had never smoked. More than half of the patients (63.7%) had a high or very high cardiovascular risk.
Table 2 presents the main characteristics of the acute period of COVID-19 in the participants of the «COVID-19 – long-term follow-up» program. During stay at the COVID center, the maximum severity of pneumonia corresponded to moderate or severe in almost half of the patients (47.6%) according to the chest CT. More than half of the patients needed oxygen support, 6.6% had cardiovascular complications, most often AF paroxysms.
Table 2. Selected indicators of the acute period of COVID-19 in patients included in the study (n=213)

During the period of 3-7 months after discharge from the COVID center and before inclusion in this study, 17 (8%) patients were hospitalized, with more than half of the patients (10 out of 17) were hospitalized for CVD. 11 (64.7%) out of 17 hospitalizations were planned and 6 (35.3%) hospitalizations were emergency (2 due to AF paroxysms, 2 due to hypertensive crisis, 1 due to severe anemia, 1 due to exacerbation of urolithiasis).
Every tenth patient (11.6%, 18 out of 155) with the initial presence of hypertension noted a worsening of its course after recovery. Another 4 (4.2%) patients with initially normal levels of blood pressure during the observation period were diagnosed with hypertension, which required hospitalization due to a hypertensive crisis in 2 cases. Prior to hospitalization, coronary artery disease was present in 27 (12.7%) patients, of which 2 patients in the convalescent period had the appearance/resumption of angina attacks. New cases of CAD were identified in 4 (1.9%) patients, one patient (0.5%) had an ischemic stroke. For the first time, cardiac arrhythmias in the form of extrasystoles were observed in 34 (16.4%) patients. Other diseases (n=6; 2.8%) included 2 cases (0.9%) of exacerbation of arthrosis of the knee and hip joints, 2 cases (0.9%) of severe exacerbation of cholelithiasis, 1 case of severe progression of myopia and 1 case of decompensation type 2 diabetes mellitus.

Figure 1. Frequency of patient complaints in the post-COVID period (n=213)
At the time of the survey, 114 (53.5%) patients previously hospitalized for a new coronavirus infection had any complaints within the framework of the «COVID-19 – long-term follow-up» program (Fig. 1). The most frequent complaints were shortness of breath and general weakness. Every tenth patient (11.3%) complained of pain in the region of the heart, while we couldn't exclude the presence of angina pectoris in 6 (2.8%) patients with chest pain.
As we noted above, every third patient (33%) complained of shortness of breath during a medical examination. An even higher frequency of dyspnea in the examined patients was established according to the mMRC dyspnea severity scale – dyspnea of varying severity was present in 59% of patients in the post-COVID period (it was mild in 47.2% of cases, moderate in 8% of cases and severe in 2, 8% of cases).
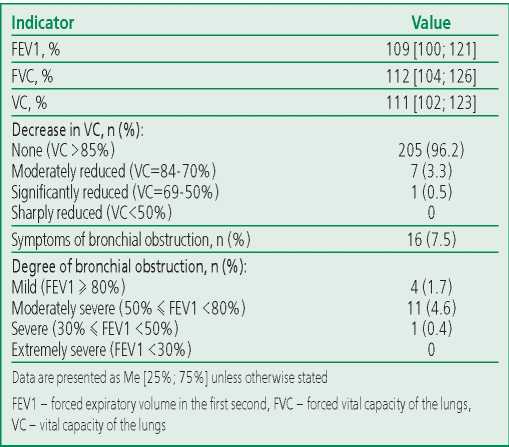
Almost every second (47.6%) patient included in the «COVID-19 - long-term follow-up» program during the index hospitalization had a fairly pronounced lung injury (CT 3-4), but nevertheless, according to spirometry, the vast majority of patients had vital capacity (VC) within normal limits. A moderate decrease in VC was observed in only 3.3% of participants, a significant decrease was noted in 0.5%. Signs of bronchial obstruction, mostly mild (FEV1 ≥ 80%) and moderate (50% ≤ FEV1 <80%), occurred in 7.5% of patients (Table 3).
Table 3. Spirometry data in patients included in the study (n=213)
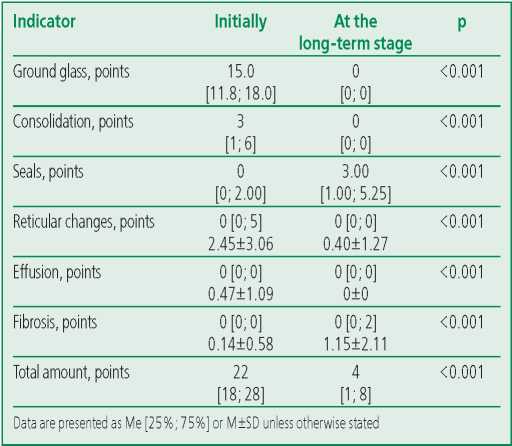
Chest CT scan was performed in 78 patients, in whom the maximum severity of lung damage during hospitalization corresponded to grades 3-4. The acute phase of the disease in all patients appeared in areas of ground glass (100%), in the majority - in areas of consolidation (80.8%), in almost half - in reticular changes (47.4%), in a third of patients - in linear and small nodular seals (34.6%), in 6.4% - in fibrosis. In the long-term period, every tenth (10.8%) patient who had severe lung damage in the acute phase of the disease, had changes in the lungs typical of COVID-19 in the form of «ground glass» persisted at the long-term stage, fibrotic changes formed in 35.9% patients, and linear and small focal seals were present in 76.9% of patients. Table 4 presents the severity of the identified changes. The total volume of lung tissue damage over the past period has significantly decreased: the median of the total point decreased from 22 [ 18; 28] to 4 [ 1; 8] (p<0.001). At the same time, the median points for ground glass, consolidation, and reticular changes also decreased. The median points for lung tissue compaction and fibrosis increased. None of the participants had a pleural effusion on long-term followup.
Table 4. Results of multislice computed tomography of the chest organs (CT-score distribution; n=78)
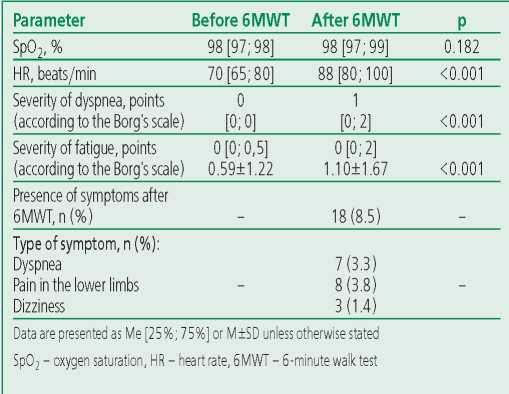
The «COVID-19 – long-term follow-up» program included the objectification of exercise tolerance using 6MWT. The median distance passed by patients was 480 [420; 500] m (maximum 700 m, minimum 250 m). The calculations of the predicted 6MWT distance showed that the predicted 6MWT distance in men was 590.3±8.7 m, and the distance passed by patients was 477.2±65.5 m, and 482.3±78.3 and 442.5±86.1 m, respectively, in women. After 6MWT, 8.5% of participants experienced any symptoms (mostly shortness of breath, dizziness, pain in the lower extremities). At the same time, patients noted an increase in heart rate, dyspnea, and fatigue on the Borg's scale, but saturation remained practically unchanged (Table 5). 26 (12.3%) patients passed less than 70% of the 6MWT distance, 142 (67%) patients passed 71-99% of the distance and 44 (20.7%) passed 100% or more of the distance.
Table 5. Results of the six-minute walk test in patients (n=212)
Discussion
This study allowed us to assess the long-term consequences of COVID-19 in hospitalized patients over a 3-7 month follow-up period. First of all, we note that despite the small average age (56.8±12.5 years) of the random sequential sample of patients that were included in the present study, 27 (4.4%) patients died in a short period (3– 7 months) after hospitalization for COVID-19, of which 26 (96.3%) had CVD. Rehospitalization was required in 8 (17%) patients, with more than half (58.8%; 10 out of 17) of patients hospitalized for CVD.
A similar overall mortality rate of 4.8% at the long-term follow-up (90 days) after discharge of patients with COVID-19 from the hospital was established in the CORE-registry (4,906 patients, average age was 61.7 years, 53.7% men) [17]. Several studies have shown even higher long-term mortality and readmission rates. For example, according to a large UK study of 47,780 patients (average age was 65 years, 55% men) discharged alive after hospitalization for COVID-19, nearly a third of patients (29.4%; n=14,060) needed readmissions for the follow-up period of 140 days, and deaths occurred in 12.3% (n=5875) of patients [18]. Dutch investigators report a readmission rate of 11.7% and a mortality rate of 6.4% over an average follow-up period of 80 days after discharge [19]. The main reasons for rehospitalization were respiratory failure (31%), arterial and venous thrombosis (16%) or diseases associated with chronic comorbidities (14%). Mortality was higher in elderly patients and patients who developed delirium during their hospital stay. Risk factors for rehospitalization were male gender, discharge to rehabilitation facilities, and chronic obstructive pulmonary disease [19].
Every tenth patient (11.6%) with the initial presence of hypertension included in our study noted a worsening of its course in the post-COVID period. New cases of hypertension were detected in 4.2%, new cases of coronary artery disease were detected in 1.9% of patients. According to British colleagues, new cases of cardiovascular complications (myocardial infarction, cerebral stroke, death from CVD), type 2 diabetes mellitus and chronic kidney disease were recorded in 4.8%, 4.9% and 1.5% of cases, respectively [18].
According to a large meta-analysis of 57 studies including 250,351 patients (average was age 54.4 years, 56% men, 79% hospitalized), a new coronavirus infection COVID-19 has some kind of longterm consequences in every second (54%) patient 6 or more months after the acute period of the disease. The most commonly reported outcomes included respiratory failure, residual changes on CT scans, psychoemotional disturbances, problems concentrating, and reduced physical performance [20].
According to our data, 114 (53.5%) patients hospitalized for a new coronavirus infection had any complaints at the time of examination under the «COVID-19 – long-term follow-up» program. Every third patient (33%) complained of dyspnea. An even higher incidence of dyspnea in the post-COVID period in the examined patients was established according to the mMRC dyspnea severity scale: 59% had dyspnea of varying severity (it was mild in 47.2%, moderate in 8% and severe in 2.8%). Almost as often as shortness of breath, patients noted weakness (27.4%). According to an Italian study conducted in the province of Bergamo, 22.8% of patients had moderate to severe dyspnea on the mMRC scale after an average of 81 days after hospitalization, while every second patient (51.4%) presented a wide range of complaints during examination, most often for dyspnea and weakness, and every third was in a post traumatic stress state [21]. Persistence of dyspnea, weakness, and psycho-emotional distress for a long period after COVID-19 turned out to be typical of a new coronavirus infection, and it was also characteristic of acute respiratory syndrome SARS. Canadian researchers reported that they observed almost complete recovery of physical performance in survivors 1 year after SARS, while a third (33%) of patients continued to have mental distress [22].
Every tenth of our patients (11.3%) complained of pain in the region of the heart, while chest pain in 2.8% of patients didn't allow us to exclude the presence of angina pectoris. Feelings of interruptions in the work of the heart were noted in 8.5% of cases. We rarely received complaints of hair loss (6.1%), cough (3.8%), memory loss (2.8%), hearing loss (1.9%), visual acuity (1.4%), attention (0.9%) and loss of smell (0.9%). Obviously, the nature of patient complaints at the follow-up long-term stage after COVID-19 has some local specifics. For example, in a Chinese study involving 1733 patients, 6 months after discharge from the hospital, 76% of patients complained of any complaints, 63% complained of weakness, 26% complained of sleep disturbances, 22% complained of hair loss, 11% complained of smell disturbance, 9% complained of interruptions in the work of the heart, 9% complained of joint pain, 8% complained of decreased appetite, 7% complained of taste disturbances, 6% complained of dizziness, 5% complained of nausea and vomiting, 5% complained of heart pain, 4% complained of sore throat, 3% complained of skin rashes, 2% complained of myalgia, and another 2% complained of headaches [23]. We will also note the multiplicity of symptoms reported by patients. For example, according to A. Carfi et al [24], after an average of 60 days after COVID-19, 1-2 symptoms bothered 32%, ≥3 symptoms bothered 55% of patients. And in our study, 38.5% of patients had 1-2 complaints, and 15.0% had ≥3 complaints. The severity of symptoms in the post-COVID period demonstrates dependence on the severity of the disease course in its acute phase [25].
Almost every second (47.6%) patient included in the "COVID-19 – long-term follow-up" program during the desired hospitalization had a fairly pronounced lung injury (CT 3-4), but nevertheless, according to spirometry, VC were within normal limits in the vast majority patients. A decrease in VC to moderate severity was noted in only 3.3% of participants, a decrease to severe severity was noted in 0.5%. Signs of bronchial obstruction, mostly mild and moderate, were present in 7.5% of patients.
Other authors also come to similar conclusions [23]. Korean researchers found a return to normal spirometry in 82% of patients 3 months after discharge [26]. According to our data, «ground-glass» changes in the lungs typical of COVID-19 persist at the longterm follow-up stage in every tenth (10.8%) patient who had severe lung damage in the acute phase of the disease; fibrotic changes form in 35.9 % of patients, and linear and small focal seals are present in 79.6% of patients. Very similar results were obtained in a study by R.F. D'Cruz et al (median follow-up of patients after hospitalization was 61 days, average age was 59 years, 62% were men): 75% of patients observed interstitial changes in the lung tissue and only 13% retained ground-glass seals [27]. A relationship was shown between the number of days spent on oxygen support in the acute phase of the disease and the severity of chest CT changes at a long-term stage [28].
The median distance passed by patients was 480 [ 420; 500] m, which corresponded to the lower part of the normal range of 400-700 m. Conducting 6MWT didn't cause significant discomfort in most participants: the appearance of any symptoms was noted in only 8.5% of patients (mainly dyspnea, dizziness and pain in the lower extremities). However, only every fifth participant managed to pass 100% or more of the 6MWT predicted distance; two-thirds
of patients passed 71% to 99% of the predicted distance, and 12.3% of patients passed less than 70% of the 6MWT predicted distance. At the same time, according to C. Huang et al. [23], six months after COVID-19, the median percentage of the 6MWT predicted distance, calculated using the same formula as in our study, was more than 85% even in the initially most severe categories patients. Perhaps the difference in the results is due to the ethnic characteristics of the examined patients.
Study limitations. According to modern international recommendations, we should use those formulas that have been validated in the local population or as close as possible to it [13] to calculate the 6MWT predicted distance, but at the moment there are no formulas for calculating the 6MWT predicted distance validated for the Russian population. In addition to the problem with interpretation of 6MWT results noted above, the limitations of this study include the refusal of face-to-face visits, mainly due to social restrictions, among the older patients contacted by telephone. Also, the design of the study did not imply the presence of a control group, which does not make it possible to draw final conclusions that the findings found in the participants are the result of the past infection, and not some unaccounted for factors.
Conclusion
The results of this study indicate the presence of long-term negative consequences of a new coronavirus infection COVID-19 in more than half of hospitalized patients. The number of recoveries of COVID19 is so high that the presence of health problems in the long-term follow-up stage, even for some of them, can provoke another health crisis associated with the need to provide medical care to a very large number of chronically ill patients. All this dictates, on the one hand, the importance of intensifying measures to prevent the development of the disease through vaccination, on the other hand, conducting further research on monitoring patients who have undergone COVID-19 in order to develop rehabilitation and dispensary care programs, especially for people with a high risk of complications, to which include patients with CVD.
Relationships and Activities. None.
Funding: The study was performed with the support of the National Medical Research Center of Cardiology named after Academician E.I.Chazov.
References
1. Ghebreyesus TA. WHO Director-General's opening remarks at the media briefing on COVID-19. 11 March 2020 [cited 2022 Jan 10]. Available from: https://www.who.int/ru/directorgeneral/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid19---11-march-2020.
2. WHO Coronavirus (COVID-19) Dashboard [cited 2022 Jan 10]. Available from: https://covid19.who.int/.
3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. DOI:10.1016/S0140-6736(20)30183-5.
4. Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-42. DOI:10.1001/jama.2020.2648.
5. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531e. DOI:10.1007/s00392-020-01626-9.
6. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052-9. DOI:10.1001/jama.2020.6775.
7. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. DOI:10.1136/bmj.m1966.
8. Boytsov SA, Pogosova NV, Paleev FN, et al. Clinical Characteristics and Factors Associated with Poor Outcomes in Hospitalized Patients with Novel Coronavirus Infection COVID-19. Kardiologiia. 2021;61(2):4-14 (In Russ.) [Бойцов С.А., Погосова Н.В., Палеев Ф.Н., и др. Клиническая картина и факторы, ассоциированные с неблагоприятными исходами у госпитализированных пациентов с новой коронавирусной инфекцией COVID-19. Кардиология. 2021;61(2):4-14]. DOI:10.18087/cardio.2021.2.n1532.
9. Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronavirus (MERS) outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063. DOI:10.2340/16501977-2694.
10. Gaman SA, Ternovoy SK, Pogosova NV, et al. Delayed CT scan of the lungs in patients with COVID19 pneumonia. REJR. 2021;11(1):8-14 (In Russ.) [Гаман С.А., Терновой С.К., Погосова Н.В., и др. Отсроченная КТ легких у пациентов, перенесших COVID-19 пневмонию. REJR. 2021;11(1):8-14]. DOI:10.21569/2222-7415-2021-11-1-8-14.
11. Perez T, Burgel PR, Paillasseur J, et al. Modified Medical Research Council scale vs Baseline Dyspnea Index to evaluate dyspnea in chronic obstructive pulmonary disease. Int J Chron Obst Pulm Disease. 2015;10(1):1663-72. DOI:10.2147/COPD.S82408.
12. "Temporary Methodological Guideline "Medical Rehabilitation for New Coronavirus Infection (COVID19). Version 2 (31.07.2020)" (approved by the Ministry of Health of Russia) [cited 2022 Jan 10]. Available from: https://xn--80aesfpebagmfblc0a.xn--p1ai/ai/doc/461/attach/28052020_Preg_ COVID-19_v1.pdf (In Russ.) ["Временные методические рекомендации "Медицинская реабилитация при новой коронавирусной инфекции (COVID-19). Версия 2 (31.07.2020)" (утверждены Минздравом России) [цитировано 10.01.2022]. Доступно на: https://xn--80aesfpebagmfblc0a.xn--p1ai/ai/doc/461/attach/28052020_Preg_COVID-19_v1.pdf].
13. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-46. DOI:10.1183/09031936.00150314.
14. Bubnova MG, Persiyanova-Dubrova AL. Six-minute walk test in cardiac rehabilitation. Cardiovascular Therapy and Prevention. 2020;19(4):2561 (In Russ.) [Бубнова М.Г., Персиянова-Дуброва А.Л. Применение теста с шестиминутной ходьбой в кардиореабилитации. Кардиоваскулярная Терапия и Профилактика. 2020;19(4):2561]. DOI:10.15829/1728-8800-2020-2561.
15. Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384-7. DOI:10.1164/ajrccm.158.5.9710086.
16. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-81. DOI: 10.1093/eurheartj/ehw106.
17. Giannis D, Allen SL, Tsang J, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137(20):2838-47. DOI:10.1182/blood.2020010529.
18. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. DOI:10.1136/bmj.n693.
19. Leijte WT, Wagemaker NMM, van Kraaij TD, et al. Mortality and re-admission after hospitalization with COVID-19. Ned Tijdschr Geneeskd. 2020;164:D5423.
20. Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARSCoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. DOI:10.1001/jamanetworkopen.2021.28568.
21. Venturelli S, Benatti SV, Casati M, et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149:e32. DOI:10.1017/S0950268821000145.
22. Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167(12):1312-20. DOI:10.1001/archinte.167.12.1312.
23. Huang С, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-32 DOI:10.1016/S0140-6736(20) 32656-8.
24. Carfì A, Bernabei R, Landi F, et al. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020; 324(6):603-5. DOI:10.1001/jama.2020.12603.
25. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013-22. DOI:10.1002/jmv.26368.
26. Liang L, Yang B, Jiang N, et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020;35(47):e418. DOI:10.3346/jkms.2020.35.e418.
27. D’Cruz RF, Waller MD, Perrin F, et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021;7(1):00655-02020. DOI:10.1183/23120541.00655-2020.
28. Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID19-related hospitalisations. Thorax. 2021;76(4):402-4. DOI:10.1136/thoraxjnl-2020-216308.
About the Authors
N. V. PogosovaRussian Federation
Nana V. Pogosova
Moscow
F. N. Paleev
Russian Federation
Filipp N. Paleev
Moscow
A. K. Ausheva
Russian Federation
Aza K. Ausheva
Moscow
D. T. Kuchiev
Russian Federation
David T. Kuchiev
Moscow
S. A. Gaman
Russian Federation
Svetlana A. Gaman
Moscow
T. N. Veselova
Russian Federation
Tatiana N. Veselova
Moscow
M. B. Belkind
Russian Federation
Mikhail B. Belkind
Moscow
O. Yu. Sokolova
Russian Federation
Olga Yu. Sokolova
Moscow
R. A. Zhetisheva
Russian Federation
Radima A. Zhetisheva
Moscow
S. K. Ternovoy
Russian Federation
Sergey K. Ternovoy
Moscow
S. A. Boytsov
Russian Federation
Sergey A. Boytsov
Moscow
Review
For citations:
Pogosova N.V., Paleev F.N., Ausheva A.K., Kuchiev D.T., Gaman S.A., Veselova T.N., Belkind M.B., Sokolova O.Yu., Zhetisheva R.A., Ternovoy S.K., Boytsov S.A. Sequelae of COVID-19 at long-term follow-up after hospitalization. Rational Pharmacotherapy in Cardiology. 2022;18(2):118-126. https://doi.org/10.20996/1819-6446-2022-04-03
















































