Scroll to:
Neuregulin-1β, Biomarkers of Inflammation and Myocardial Fibrosis in Heart Failure Patients
https://doi.org/10.20996/1819-6446-2022-09-05
Abstract
Neuregulin-1β (NRG-1) is an emerging biomarker of heart failure (HF). The mechanisms of its action in HF patients are yet to be investigated. Cardioprotective and anti-inflammatory effects of NRG-1 have been reported.
Aim. To assess NRG-1 levels in HF patients and investigate the association between NRG-1 and biomarkers of inflammation and myocardial fibrosis.
Material and Methods. NRG-1, biomarkers of inflammation and fibrosis (hsCRP, IL-6, sVCAM-1, MMP-9, Galectin-3, ST2, TGF-β) were assessed in 47 patients with HF and preserved ejection fraction (HFpEF); 39 patients with HF and reduced ejection (HFrEF) and 40 healthy participants. The associations between NRG-1 and biomarkers of inflammation and fibrosis, as well as the composite outcomes of cardiovascular death and HF hospitalisations were assessed.
Results. Median NRG-1 levels in HFpEF were 0.969 (0.348; 1.932) ng/ml, in HFrEF – 0.63 (0.348; 1.932), in healthy participants 0.379 (0.195; 0.861) ng/ml, and was significantly higher in HFpEF compared to healthy volunteers (р=0.004). There was no difference in NRG-1 concentration between HFpEF and HFrEF. In HF patients, all biomarkers of inflammation and fibrosis were higher than in controls. ST2, IL-6 and TGF-β were significantly higher in HFrEF compared to HFpEF patients, while hsCRP, sVCAM-1, MMP-9, and Galectin-3 levels were comparable. In HFpEF, NRG-1 was associated with hsCRP (rs=0.378, p=0.023) and IL-6 (rs=0.378, p=0.014). Median follow-up time in patients with HFpEF and in patients was 312 (236; 388) days, in HFrEF – 147 (98; 237) days. In HFpEF, 2 patients died and 19 were hospitalized due to HF. In HFrEF, 10 deaths and 19 hospitalizations were registered. Kaplan-Mayer analysis showed that HFpEF patients with increased NRG-1 and IL-6 had higher levels of HF hospitalisation (log rank test, р=0.046 and р=0.012, respectively). In a multivariable cox proportional hazard model, the association between the NRG-1 and outcomes remained significant after adjustment for age, gender and NTproBNP but diminished when hsCRP and IL-6 were included in the model.
Conclusion. NGR-1 level significantly higher in HFpEF compared to healthy participants, and comparable with NRG-1 concentrations in HFrEF. In HFpEF, NRG-1 was associated with biomarkers of inflammation and fibrosis. The prognostic value of NRG-1 in HF requires further investigations.
Keywords
For citations:
Zhbanov K.A., Salakheeva E.Yu., Sokolova I.Ya., Zheleznykh E.A., Zektser V.Yu., Privalova E.V., Belenkov Yu.N., Shchendrygina A.A. Neuregulin-1β, Biomarkers of Inflammation and Myocardial Fibrosis in Heart Failure Patients. Rational Pharmacotherapy in Cardiology. 2022;18(5):522-529. https://doi.org/10.20996/1819-6446-2022-09-05
Introduction
High morbidity and mortality from chronic heart failure (CHF) determine the need for further improvement of approaches to the diagnosis and treatment of this condition [1]. Neuregulin-1β is an endothelial growth factor that is produced by endotheliocytes of coronary microvessels in response to oxidative stress and ischemic injury [2-4]. Neuregulin-1β activates tyrosine kinase receptors of types 3 and 4 on the membrane of cardiomyocytes, fibroblasts, and coronary endothelium, triggering a cascade of adaptive intracellular reactions, which leads to an increase in the resistance of cardiomyocytes to oxidative stress and apoptosis [2][4][5]. Neuregulin-1β is currently being studied as biomarker for heart failure. Several studies have demonstrated its predictive value [6][7]. Changes in the level of neuregulin-1β were noted in patients with CHF with reduced ejection fraction (HFrEF) already in the early stages of the disease [8]. In addition, studies have shown that the administration of recombinant neuregulin-1β to patients with HFrEF leads to an improvement in left ventricular (LV) systolic function and reverse myocardial remodeling [8]. The mechanisms of the cardioprotective effects of neuregulin-1β in CHF are yet to be investigated.
A significant role of subclinical systemic inflammation, endothelial dysfunction of coronary microvessels, and myocardial fibrosis in the development and progression of CHF has been demonstrated both in patients with HFrEF and in CHF patients with preserved ejection fraction (HFpEF) [9][10]. Recent studies indicate anti-inflammatory and antifibrotic effects of neuregulin-1β [11-16]. The association between the level of neuregulin-1β and markers of systemic inflammation and fibrosis in patients with CHF has not been previously assessed.
The aim of the study was to assess the plasma concentration of neuregulin-1β in patients with CHF and investigate the association of neuregulin-1β with markers of systemic inflammation and myocardial fibrosis, as well as to investigate the associations of the studied biomarkers with clinical outcomes.
Materials and Methods
A prospective observational study was conducted in accordance with the recommendations of STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) [17] and the principles of the Declaration of Helsinki.
The study included 86 patients with CHF [patients with HFpEF (n=47), patients with HFrEF (n=39)] and healthy volunteers (control; n=40). The study was conducted at the cardiology department of the University Clinical Hospital No. 1 (Sechenov University) from September 2019 to June 2020.
Inclusion criteria for patients with HFpEF were as following: CHF symptoms (NYHA class II-IV); left ventricular ejection fraction >50%; brain terminal natriuretic peptide (NT-proBNP) >300 pg/ml; the presence of structural changes in the heart according to echocardiography: left ventricular hypertrophy (the thickness of the posterior LV wall ≥12 mm and/or an increase in the left atrium (LA), and/or an increase in the index of the left atrium >34 ml/m2, and / or left ventricular mass index >115 g/m2 for men and 95 g/m2 for women) [18].
Inclusion criteria for patients with HFrEF: CHF symptoms (NYHA class II-IV) and left ventricular ejection fraction <40% according to echocardiography [18].
Exclusion criteria: acute coronary heart disease (CHD); stroke within 3 months prior to study entry; chronic obstructive pulmonary disease stage 3-4; severe liver dysfunction; exacerbation of chronic diseases of the gastrointestinal tract; acute renal failure; type 1 diabetes; hypothyroidism or hyperthyroidism in the stage of decompensation; autoimmune diseases; viral hepatitis B and C, carriage of HIV infection, oncological diseases.
The group of healthy volunteers included persons over 50 years of age who had no cardiovascular diseases (CVD) and CVD risk factors.
Patients with CHF were followed up for 2 years. The number of cases of cardiovascular death (CVD) and hospitalization for decompensation of CHF was recorded.
Clinical Data
Demographic and clinical parameters including gender, age, weight, height, body mass index (BMI), CVD risk factors, and NYHA class. Were recorded in the patient's individual electronic card in the RedCAP system.
Biomarkers
Venous blood samples of patients was taken at the time of inclusion. After centrifugation for 20 min, plasma samples were frozen at 2000 g and stored at -80°C. Assessment of the concentration of neuregulin-1β, matrix metalloproteinase-9 (MMP-9), galectin-3, soluble vascular endothelial adhesion molecule type 1 (sVCAM-1), transforming growth factor β (TGF-β), stimulating growth factor (ST-2) was carried out by ELISA method using the following kits: NRG-1 Duoset ELISA (R&D Systems®, USA), coefficient of variation (CV) within series was 6.1%, between analyzes was 13.8%; MMP-9 Human ELISA Kit (Invitrogen, Thermo Fisher, USA), CV within series was was 7.3%, between analyzes was 10.2%; Galectin-3 Human ELISA Kit (Invitrogen, Thermo Fisher, USA), CV within series was 5.4%, between analyzes was 7.5%; VCAM-1 (Soluble) Human ELISA Kit (Invitrogen, Thermo Fisher, USA), CV within series was 5.2%, between analyzes was 3.1%; TGF beta-1 Human ELISA Kit (Invitrogen, ThermoFisher, USA), CV within series was 4.9%, between analyzes was 3.2%; Presage® ST2 Assay+Control kit (Critical Diagnostics, USA) CV within series was 6.3%, between analyzes was 4.8%. The analysis results were recorded on a microplate reader (Luminometer Photometer LMA0B Beckman Coulter, 450 nm), data processing was performed using the 5PL algorithm.
Interleukin-6 (IL-6) and N-terminal B-type natriuretic hormone propeptide (NT-proBNP) concentrations were determined by immunoassay using ElecsysproBNP II (Roche, Switzerland) and Elecsys IL-6 (Roche, Switzerland) kits, respectively. The results were recorded automatically using a CobaseE 601 analyzer (Roche, Switzerland). Quantitative determination of the level of high-sensitive C-reactive protein (hsCRP) in blood plasma was carried out by latex-enhanced immunonephelometric method using a BN ProSpec automatic analyzer manufactured by Dade-Behring, Germany, using the CardioPhasehs CRP reagent (Siemens, USA).
Echocardiographic Study
Echocardiography was performed on a Toshiba Apilo 500 device in accordance with recommendations for echocardiography [19]. The parameters of the area and volumes of the left and right atria, the end systolic and diastolic volume of the left ventricle (LV), LV wall thickness and its mass were assessed. Volumetric parameters were indexed to body surface area. The LV ejection fraction was measured using the Simpson's method. LV diastolic function was assessed using the methods of pulse and tissue doppler with calculation of the E/e' parameter. Valvular hemodynamics were also assessed.
Statistical analysis
Normality of the distribution tested out using the Shapiro-Wilk's test. Continuous variables presented as mean SD ± standard deviation if normally distributed, or otherwise as median and interquartile range (IQR). Categorical variables parameters are presented as absolute values and percentages. Chi-square test or Mann-Whitney's U-test was used for pairwise comparisons. One-way analysis of variance using the Tukey's or Games-Howell's test was performed to assess differences in the three groups with a normal distribution of the indicator. The Kruskal-Wallis's test was used with a non-normal distribution. Correlations were assessed using Spearman's rank correlation coefficient. Differences were considered statistically significant at p<0.05. Kaplan-Meier's curves were constructed to compare the time of onset of events in subgroups of patients with high and low levels of biomarkers. The median concentration of these biomarkers obtained in the study cohort of patients with CHF was used for the Kaplan-Meier analysis as reference values for neuregulin-1β, IL-6, sVCAM-1, MMP-9 and TGF-β. 2 mg/l served as a reference value for hsCRP [20], 23 ng/ml for ST-2 [21], 17.8 ng/ml for galectin-3 [22]. These are values whose predictive value has been established in previous studies. Differences were assessed using the log rank test. Cox's regression was used to assess the association between studied biomarkers and outcomes. Statistical data processing was carried out using the Statistica 10.0 statistical software package (Statsoft Inc., USA) and GraphPad Prism 8 Software (GraphPad Software Inc., USA).
Results
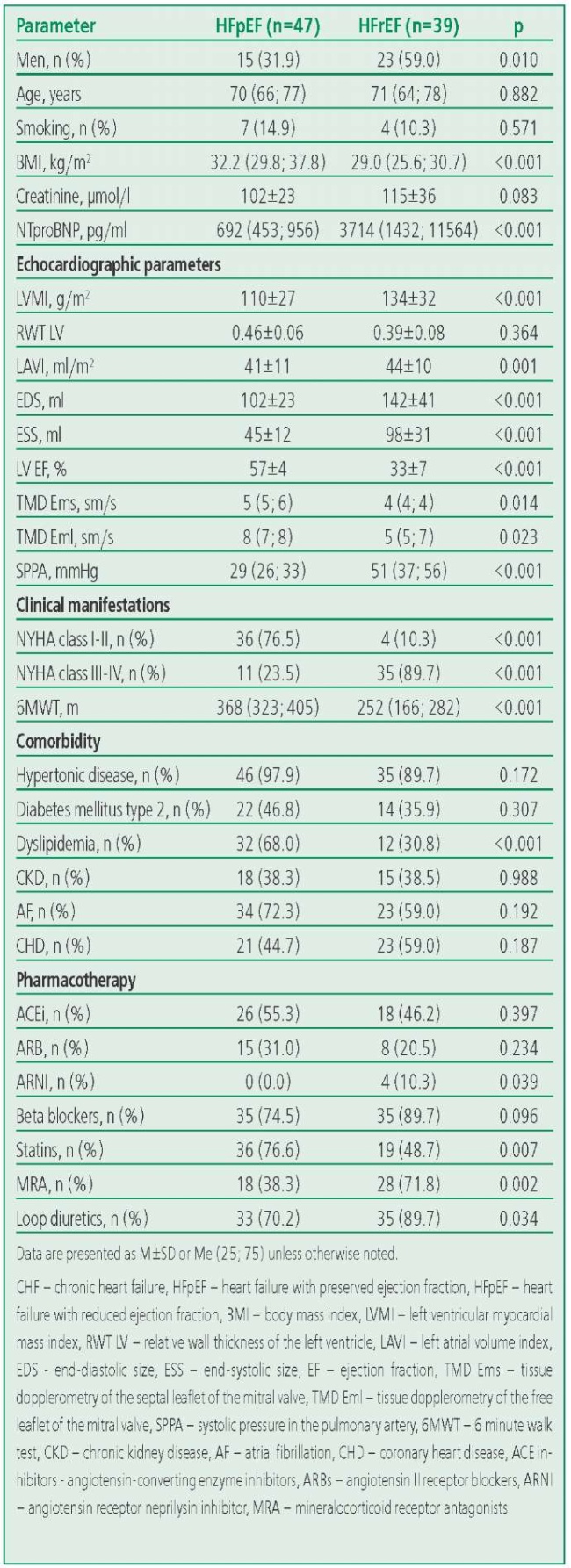
The main characteristics of the groups are presented in Table 1. In HFrEF group were predominantly by men (n=23; 59%), while the HFpEF group was dominated by women (n=32; 68%). Patients in the HFrEF and HFpEF groups were comparable in age and the presence of comorbidities. Patients with HFrEF were more likely than patients with HFpEF to receive loop diuretics and mineralocorticoid receptor antagonists (p=0.034 and p=0.002, respectively). There were no differences in the frequency of prescribing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, and beta-blockers in the CHF groups. The proportion of patients with NYHA class II was higher in the HFpEF group. The age of healthy volunteers (n=40; 45% men) was 56 (53; 61) years.
Table 1. Clinical and Demographic Characteristics of Patients
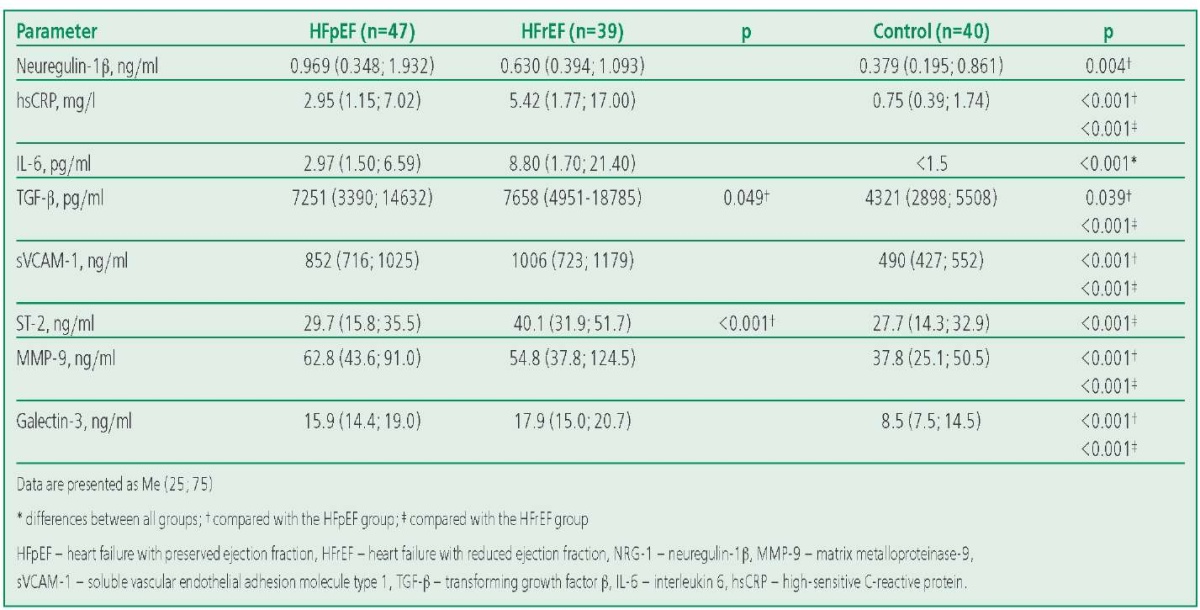
The concentration of neuregulin-1β in the HFpEF group didn't differ significantly from that in the HFrEF group, but was significantly higher than in the control group (Table 2). The levels of all studied biomarkers of systemic inflammation (IL-6, sVCAM-1, TGF-β and ST2) were significantly higher in patients with CHF than in those in the control group. Concentrations of ST-2, TGF-β and IL-6 were significantly higher in patients with HFrEF than in patients with HFpEF. At the same time, hsCRP and sVCAM-1 in the groups of patients with CHF were comparable (p>0.05). The levels of fibrosis biomarkers MMP-9 and galectin-3 didn't differ between the HFpEF and HFrEF groups (p>0.05), but were significantly higher in both CHF groups compared to the control group (p<0.01).
Table 2. Concentrations of Neuregulin-1β and Biomarkers of Systemic Inflammation and Fibrosis in the Study Groups
In the HFrEF group, the levels of systemic inflammatory biomarkers were significantly higher in NYHA class III-IV compared with I-II: hsCRP [ 6 (2; 21) vs. 1.04 (0.75; 2.57) mg/l ; p=0.012], IL-6 [ 10.1 (3.8; 25.5) vs. <1.50 pg/ml (less than the analyzer detection threshold); p=0.007], ST-2 [ 41 (32; 53) vs. 31 (29; 32) ng/mL; p=0.042], while the level of neuregulin-1β didn't differ significantly. In the group of HFpEF individuals, there were no differences in the level of biomarkers depending on the NYHA class of CHF (Table P1).
In the HFpEF group, a significant moderate correlation between the levels of neuregulin-1β and biomarkers of systemic inflammation (hsCRP; IL-6) and fibrosis (TGF-β) was seen (Fig. 1). Correlations of the level of neuregulin-1β with demographic and laboratory characteristics and biomarkers of inflammation and fibrosis are presented in Table P2.
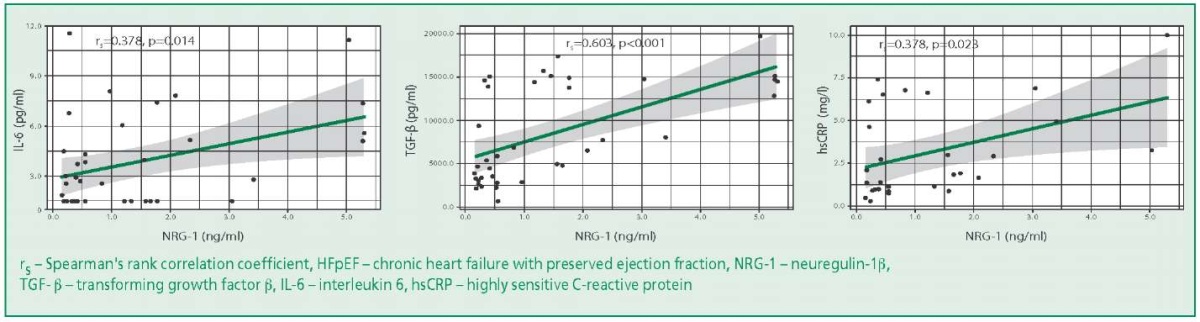
Figure 1. Graphs of Regression Functions Characterizing the Dependence of IL-6, hsCRP, TGF-β levels on NRG-1 level in the HFpEF
In the HFpEF group, 2 (4%) deaths from CVD and 19 (40%) hospitalizations for HF were registered within 456 (240; 730) days follow-up. In the HFrEF group, during the follow-up period of 407 (98; 730) days, 10 (25%) patients died and 19 (48%) patients were hospitalized for decompensated CHF.
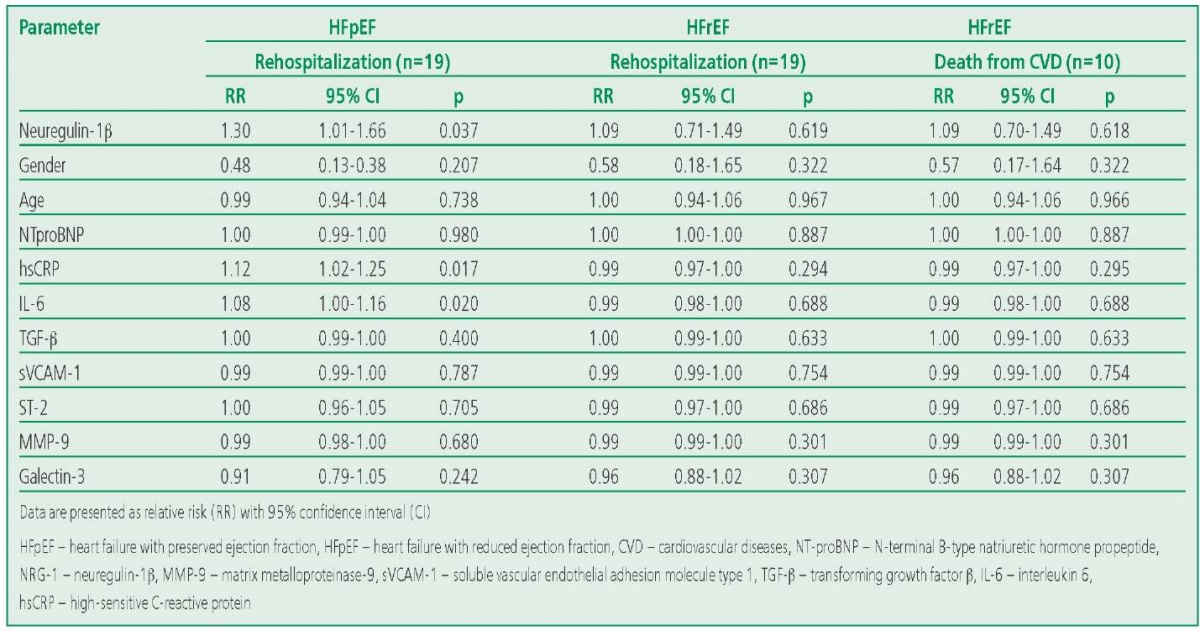
Kaplan-Meier analysis showed that the frequency of HF was significantly higher in patients with HFpEF with elevated levels of neuregulin-1β than in those whose biomarker values remained low (11 readmissions vs. 8; p=0.046). Patients with HFpEF and high levels of IL-6 also had a significantly higher rate of events (14 readmissions vs. 5 in patients with low concentrations (p=0.012) (Fig. P1)). In the group of patients with HFpEF, analysis of the association of biomarkers with this outcome was not performed due to the low level of cardiovascular mortality. A univariate regression analysis revealed that high concentrations of neuregulin-1β, IL-6, and hsCRP in patients with HFpEF were associated with a high risk of hospitalization for CHF. No such associations were found in the HFrEF group (Table 3).
Table 3. Characterization of the Factors Influencing the Risk of Hospitalizations and Death from Cardiovascular Diseases in the Study Groups
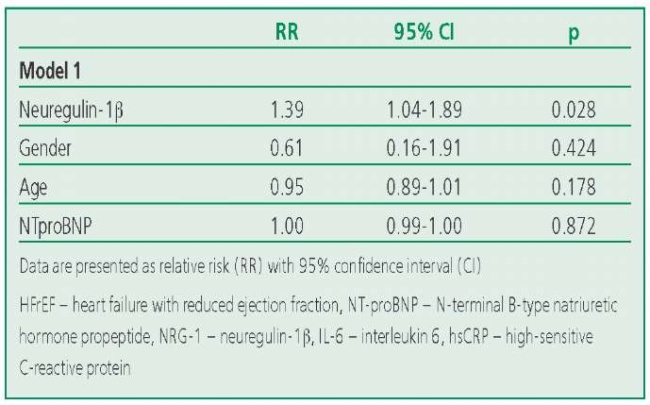
The association between the level of neuregulin-1β and risk of hospitalization remained significant after gender, age, and NTproBNP levels were included in the model (p=0.028) (Table 4).
Table 4. Characteristics of the Factors Included in the Risk Assessment Models for Hospitalization in the HFpEF Group
Discussion
In our study, we found that the concentration of neuregulin-1β was higher in patients with HFpEF than in healthy individuals. However, the level of the biomarker didn't differ significantly in HF subgroups. Our study also showed that neuregulin-1β in patients with HFpEF significantly correlated with markers of systemic inflammation. We demontrated that neuregulin-1β, hsCRP, and IL-6 were associated with a high risk of hospitalization for CHF in the HFpEF group, but not in the HFrEF group.
Previously, C. Hage et al. showed that the level of neuregulin-1β in patients with HFpEF was significantly higher than in patients with HFrEF [6]. Probably, the relatively small sample size in our study didn't allow us to identify possible differences in biomarker concentrations in HF subgroups. The reasons for the difference in the level of neuregulin-1β in HFpEF and HFrEF are not fully understood and continue to be studied. One can assume that a high level of neuregulin-1β levels in patients with HFpEF characterizes the activity of adaptive mechanisms that contribute to the resistance of cardiomyocytes to the effects of oxidative stress and apoptosis.
Lower concentrations of neuregulin-1β in patients with HFrEF may be associated with severe dysfunction of the coronary microvessels [23-27], in which the production of neuregulin-1β by the endothelium progressively decreases. This assumption is also supported by data on decreased expression of mRNA that regulates the synthesis of neuregulin-1β in HFrEF [28]. These assumptions require further studies.
Our study showed for the first time a positive correlation between neuregulin-1β and markers of systemic inflammation (hsCRP, IL-6) in patients with HFpEF. Similarly to the data of previous studies, we didn't observe associations of neuregulin-1 with such markers of fibrosis as ST-2 (p=0.277), galectin-3 (p=0.752), as well as with NTproBNP (p=0.545) [6].
We found IL-6 was significantly higher in patients with HFrEF than in patients with HFpEF. The results obtained are consistent with the data of previous studies, which also demonstrated the prognostic role of IL-6 in this category of patients [29]. We also found that the level of inflammatory markers in patients with HFrEF was significantly higher in patients with severe CHF (NYHA class III-IV), while in the HFpEF group, the levels of these markers didn't differ depending on NYHA class, although they were significantly higher than in healthy volunteers. It's likely that the severity of the condition of patients with HFrEF determines the severity of systemic inflammation, while systemic inflammation in patients with HFpEF is present regardless of the severity of CHF, playing an important role in the development and progression of the disease.
The levels of myocardial fibrosis markers (MMP-9, galectin-3) were higher in patients with HF than in controls. At the same time, the concentrations of these biomarkers didn't differ in patients with HFpEF and HFrEF, which is consistent with the results of previous studies [30][31].
We demonstrate for the first time that higher concentrations of neuregulin-1β along with IL-6 in the group of patients with HFpEF were associated with a high risk of HF, while in the group of patients with HFrEF, associations of the studied biomarkers with the risk of death from CVD and the risk of repeated hospitalizations were not obtained. At the same time, the associations of neuregulin-1β and outcomes remained significant even after adjusting for gender, age, and NTproBNP levels.
We also found that it was high concentrations of neuregulin-1 that were associated with an increased risk of adverse outcomes. We can assume that the neuregulin-1 system in patients with HFpEF is activated by a positive feedback mechanism, similar to how it happens in the case of natriuretic peptide. These assumptions require further studies.
Study Limitations. We recognize that the study has several limitations, including a relatively small sample, which limits the construction of a multivariate predictive model for assessing the predictive value of a number of biomarkers. Nevertheless, this study complements our understanding of the role of neuregulin-1β in patients with CHF and forms the basis for further studies on a larger cohort of patients with CHF.
Conclusion
The concentration of neuregulin-1β is higher in patients with HFpEF than in healthy volunteers, and is comparable to that in the HFrEF group. High levels of neuregulin-1β in patients with HFpEF are associated with markers of systemic inflammation and fibrosis, as well as the risk of hospitalization for decompensated CHF.
Relationships and Activities. None.
Funding. The study was performed with the financial support of the Russian Foundation for Basic Research within the framework of scientific project №18-515-76002, scientific grant “The neuregulin-1 pathway in development and progression cardiovascular disease: identification of small molecule ErbB4 agonists and identification of patient populations that could benefit the most”.
References
1. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342-56. DOI:10.1002/ejhf.1858.
2. Lemmens K, Segers VFM, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006;281(28):19469-77. DOI:10.1074/jbc.M600399200.
3. Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: Implications for therapy of heart failure. Circulation. 2007;116(8):954-60. DOI:10.1161/CIRCULATIONAHA.107.690487.
4. Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111(10):1376-85. DOI:10.1161/CIRCRESAHA.112.267286.
5. Segers VFM, Brutsaert DL, De Keulenaer GW. Cardiac remodeling: Endothelial cells have more to say than just NO. Front Physiol. 2018;9:382. DOI:10.3389/fphys.2018.00382.
6. Hage C, Wärdell E, Linde C, et al. Circulating neuregulin 1-β in heart failure with preserved and reduced left ventricular ejection fraction. ESC Hear Fail. 2020;7(2):445-55. DOI:10.1002/ehf2.12615.
7. Ky B, Kimmel SE, Safa RN, et al. Neuregulin-1β is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120(4):310-7. DOI:10.1161/CIRCULATIONAHA.109.856310.
8. De Keulenaer GW, Feyen E, Dugaucquier L, et al. Mechanisms of the Multitasking Endothelial Protein NRG-1 as a Compensatory Factor during Chronic Heart Failure. Circ Hear Fail. 2019;12(10):1-15. DOI:10.1161/CIRCHEARTFAILURE.119.006288.
9. Simmonds SJ, Cuijpers I, Heymans S. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved. Cells. 2020;9(1):242. DOI:10.3390/cells9010242.
10. DeBerge M, Shah SJ, Wilsbacher L, Thorp EB. Macrophages in Heart Failure with Reduced versus Preserved Ejection Fraction. Trends Mol Med. 2019;25(4):328-40. DOI:10.1016/j.molmed.2019.01.002.
11. Vermeulen Z, Hervent AS, Dugaucquier L, et al. Inhibitory actions of the NRG-1/ErbB4 pathway in macrophages during tissue fibrosis in the heart, skin, and lung. Am J Physiol - Hear Circ Physiol. 2017;313(5):H934-45. DOI:10.1152/ajpheart.00206.2017.
12. Vandekerckhove L, Vermeulen Z, Liu ZZ, et al. Neuregulin-1 attenua neuregulin-1 attenuates development of nephropathy in a type 1 diabetes mouse model with high cardiovascular risk. Am J Physiol – Endocrinol Metab. 2016;310(7):E495-E504. DOI:10.1152/ajpendo.00432.2015.
13. Xu Z, Jiang J, Ford G, Ford BD. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322(2):440-6. DOI:10.1016/j.bbrc.2004.07.149.
14. Wu L, Walas S, Leung W, et al. Neuregulin1-β Decreases IL-1β-Induced Neutrophil Adhesion to Human Brain Microvascular Endothelial Cells. Transl Stroke Res. 2015;6(2):116-24. DOI:10.1007/s12975-014-0347-9.
15. Galindo CL, Kasasbeh E, Murphy A, et al. Anti‐Remodeling and Anti‐Fibrotic Effects of the Neuregulin‐1β Glial Growth Factor 2 in a Large Animal Model of Heart Failure. J Am Heart Assoc. 2014;3(5):1-22. DOI:10.1161/jaha.113.000773.
16. Gupte M, Lal H, Ahmad F, et al. Chronic Neuregulin-1Treatment Mitigates the Progression of Postmyocardial Infarction Heart Failure in the Setting of Type 1 Diabetes Mellitus by Suppressing Myocardial Apoptosis, Fibrosis, and Key Oxidant-Producing Enzymes. J Card Fail. 2017;23(12):887-99. DOI:10.1016/j.cardfail.2017.08.456.
17. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(5):S31-4. DOI:10.4103/sja.SJA_543_18.
18. Mareev VYu, Fomin IV, Ageev FT, et al. Russian Heart Failure Society, Russian Society of Cardiology. Russian Scientific Medical Society of Internal Medicine Guidelines for Heart failure: chronic (CHF) and acute decompensated (ADHF). Diagnosis, prevention and treatment. Kardiologiia. 2018;58(6S):8-158 (In Russ.) DOI:10.18087/cardio.2475.
19. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233-71. DOI:10.1093/ehjci/jev014.
20. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119-31. DOI:10.1056/nejmoa1707914.
21. Najjar E, Faxén UL, Hage C, et al. ST2 in heart failure with preserved and reduced ejection fraction. Scand Cardiovasc J. 2019;53(1):21-7. DOI:10.1080/14017431.2019.1583363.
22. Meijers WC, Januzzi JL, Defilippi C, et al. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: A pooled analysis of 3 clinical trials. Am Heart J. 2014;167(6):853-60.e4. DOI:10.1016/j.ahj.2014.02.011.
23. Van Heerebeek L, Hamdani N, Falcão-Pires I, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126(7):830-9. DOI:10.1161/CIRCULATIONAHA.111.076075.
24. Westermann D, Lindner D, Kasner M, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Hear Fail. 2011;4(1):44-52. DOI:10.1161/CIRCHEARTFAILURE.109.931451.
25. Ohtani T, Mohammed SF, Yamamoto K, et al. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33(14):1742-9. DOI:10.1093/eurheartj/ehs135.
26. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ Res. 2000;86(5):494-501. DOI:10.1161/01.RES.86.5.494.
27. Tschöpe C, Bock CT, Kasner M, et al. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation. 2005;111(7):879-886. DOI:10.1161/01.CIR.0000155615.68924.B3.
28. Munk M, Memon AA, Goetze JP, Nielsen LB, Nexo E, Sorensen BS. Hypoxia changes the expression of the epidermal growth factor (EGF) system in human hearts and cultured cardiomyocytes. PLoS One. 2012;7(7):1-10. DOI:10.1371/journal.pone.0040243.
29. Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21(8):965-73. DOI:10.1002/ejhf.1482.
30. Podzolkov V, Dragomiretskaya N, Kazadaeva A, et al. Relationships between the activity of neurohormonal systems and intracardiac hemodynamics in patients with heart failure: focus on galectin-3. Russian Journal of Cardiology. 2022;27(4):4957 (In Russ.) DOI:10.15829/1560-4071-2022-4957.
31. Musikhina NA, Petelina TI, Kostousova AI, et al. Biomarkers of inflammation in patients with myocardial infarction and heart failure with preserved and mid-range ejection fraction: 5-year prospective follow-up. Russian Journal of Cardiology. 2020;25(12):3726 (In Russ.) DOI:10.15829/1560-4071-2020-3726.
About the Authors
K. A. ZhbanovRussian Federation
Konstantin A. Zhbanov
Moscow
E. Yu. Salakheeva
Russian Federation
Ekaterina Yu. Salakheeva
Moscow
I. Ya. Sokolova
Russian Federation
Irina Ya. Sokolova
Moscow
E. A. Zheleznykh
Russian Federation
Elena A. Zheleznykh
Moscow
V. Yu. Zektser
Russian Federation
Vita Yu. Zektser
Moscow
E. V. Privalova
Russian Federation
Elena V. Privalova
Moscow
Yu. N. Belenkov
Russian Federation
Yuri N. Belenkov
Moscow
A. A. Shchendrygina
Russian Federation
Anastasia A. Shchendrygina
Moscow
Supplementary files

|
1. Приложение | |
| Subject | ||
| Type | Результаты исследования | |
Download
(121KB)
|
Indexing metadata ▾ | |

|
2. Annex | |
| Subject | ||
| Type | Исследовательские инструменты | |
Download
(98KB)
|
Indexing metadata ▾ | |
Review
For citations:
Zhbanov K.A., Salakheeva E.Yu., Sokolova I.Ya., Zheleznykh E.A., Zektser V.Yu., Privalova E.V., Belenkov Yu.N., Shchendrygina A.A. Neuregulin-1β, Biomarkers of Inflammation and Myocardial Fibrosis in Heart Failure Patients. Rational Pharmacotherapy in Cardiology. 2022;18(5):522-529. https://doi.org/10.20996/1819-6446-2022-09-05
















































