Scroll to:
Predictors and etiology of in-hospital mortality in patients with acute myocardial infarction
https://doi.org/10.20996/1819-6446-2024-3003
EDN: NHUKWW
Abstract
Aim. To identify factors and develop a clinical risk model (nomogram) for in-hospital mortality in patients with acute myocardial infarction (AMI) after timely early invasive diagnosis and revascularization of infarct-related artery (IRA).
Material and methods: We conducted a prospective, single-center observational study that included 712 consecutive patients with AMI [median age 65 (interquartile range 56-74 years), 61% were male, 47.8% with ST-elevation] who underwent coronary angiography <24 hours after symptom onset and successful endovascular revascularization of IRA. The primary endpoint was in-hospital mortality. Logistic regression analysis was used to identify independent prognostic risk factors for in-hospital mortality. Based on the multivariate analysis, a nomogram was developed to predict outcome. The discriminative ability of the nomogram was assessed by calculating the area under the receiver operating characteristic (ROC) curve.
Results. The in-hospital mortality rate was 5.06%. The most common cause of in-hospital mortality was acute heart failure (AHF, 75%), followed by myocardial rupture with cardiac tamponade (11.1%). Multivariate analysis revealed that age (odds ratio (OR) 1.07, 95% confidence intervals (CI) 1.01-1.14, p=0.027), Killip class (OR 2.95, 95% CI 1.67-5.23, p<0.001), hemoglobin at admission (OR 0.97, 95% CI 0.95-0.99, p=0.006), and left ventricular ejection fraction (LVEF) ≤36% (OR 8.87, 95% CI 2.95-26.69, p<0.001), were independent predictors of adverse outcome. The identified predictors were included a nomogram, which demonstrated excellent discrimination in predicting in-hospital mortality (area under the ROC curve = 0.949, 95% CI: 0.925-0.972, p<0.001, sensitivity: 91.3%, specificity: 89.9%) and good calibration (Hosmer-Lemeshow test, p=0.93).
Conclusions. Age, hemoglobin at admission, Killip class and left ventricular ejection fraction were independent predictors of in-hospital mortality in acute MI. The most common etiology of in-hospital mortality was AHF. The nomogram for prediction of in-hospital mortality demonstrated high prognostic potential, allowing for the identification of patients at high-risk of adverse outcome, and targeted therapeutic strategies may be needed to improve the survival of patients with acute MI.
Keywords
For citations:
Hoang T.H., Maiskov V.V., Merai I.A., Kobalava Z.D. Predictors and etiology of in-hospital mortality in patients with acute myocardial infarction. Rational Pharmacotherapy in Cardiology. 2024;20(3):278-284. https://doi.org/10.20996/1819-6446-2024-3003. EDN: NHUKWW
Introduction
Cardiovascular disease (CVD) is the most common cause of mortality worldwide and substantially contribute to loss of health and excess health system costs [1][2]. Acute myocardial infarction (MI) is a common clinical manifestation of CVD. The mortality of patients with acute MI has been decreasing over the last 50 years [3], in part because of improvement in acute MI prevention, diagnosis, and treatment [4]. In-hospital mortality has decreased from 29% in 1969 [5] to <7% today [6][7]. However, around 9 million people worldwide die after acute MI each year (in 2019, 16% of total death in the world) 1, and in-hospital mortality varies substantially across hospitals [7]. Therefore, study of factors predicting the risk of death for a particular medical institution, adapted to its material and technical base and the existing flow of patients with acute MI, is an urgent problem.
Although several risk scores of in-hospital mortality were developed for patients with AMI, including the "Global Registry of Acute Coronary Events" (GRACE), "Thrombolysis in Myocardial Infarction" (TIMI), and "Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines" (CRUSADE) scores [8-10], few included a representative sample from routine clinical care. Moreover, continued improvement in acute MI care requires periodic update to the risk models so that hospitals can assess their quality as contemporary care continues to evolve.
This study sought to determine factors associated with for in-hospital mortality in patients presenting with acute MI and develop a prognostic model for in-hospital mortality in a contemporary settings.
Material and methods
Study design and participants
A prospective, single-center observational study at Vinogradov Municipal Clinical Hospital (Moscow, Russia) was conducted. From January 2017 to December 2018, a total of 712 consecutive adults >18 years admitting with acute MI and undergoing coronary angiography (CAG) <24 hours after symptom onset were recruited. The exclusion criteria were type 3, 4, or type 5 MI. The diagnosis of acute MI was based on the Third universal definition of MI [11]. All patients provided written informed consent. The study was approved by the local Ethics Committee of RUDN University, and carried out in accordance with the Declaration of Helsinki.
Data collection and outcomes
Variables of interest included clinical characteristics, cardiovascular risk factors, comorbidities, physical examination findings, blood test results, and imaging data (electrocardiography, echocardiography, and CAG). Cardiac troponin I levels were measured using the Access 2 Immunoassay System (Beckman Coulter, USA), with 99th percentile upper reference limit being 0.02 ng/L. The GRACE 2.0 score was used to risk stratification for MI patients [12]. Anemia was defined as a hemoglobin concentration of less than 130 g/L for men or less than 120 g/L for women 2. The left ventricular ejection fraction (LVEF) was estimated by the modified biplane Simpson method [13] along with a transthoracic echocardiogram with using the Vivid 7 ultrasound system (General Electric Healthcare, USA).
The primary endpoint was all-cause death during the index hospitalization for acute MI, obtained from medical records.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 25.0 (SPSS Inc., Chicago, IL, USA) and R software (version 3.6.3). Descriptive statistics are presented as frequencies and percentage for categorical variables and as means ± standard deviation for continuous variables if variables adhering to a normal distribution or median (Me) and interquartile range (IQR) for skewed variables. Comparisons of categorical variables were performed by Chi-Square or Fisher’s exact tests, while for continuous variables by using the unpaired Student t-test and Mann-Whitney U test. Logistic regression analysis was used to identify factors associating with in-hospital mortality. Odds ratios (OR) and 95% confidence intervals (CI) were presented. The receiver operating characteristic (ROC) curve analysis was performed to determine cut-off level of continuous variables associated with in-hospital mortality. Based on the estimated factors from the multivariable logistic regression model, a nomogram was constructed to assess risk of in-hospital death ("rms" package in R). The discrimination accuracy of a nomogram was assessed the area under the ROC curve (AUC) [14]. The calibration was assessed by Hosmer-Lemeshow chi-square statistics for goodness of fit. A two-tailed p-value <0.05 were considered statistically significant.
Results
Among patients, 434 (61%) were male with median age of 65 (IQR: 56-74) years, 47.8% presented with ST elevation. During the follow-up period, 36 patients (5.06%) died.
The baseline characteristics of the patients are shown in Table 1. Compared with survivors, age, proportion of female gender, ST-elevation, coronary artery disease (CAD), diabetes mellitus, atrial fibrillation, chronic kidney disease, anemia, dyspnea, Killip class II-IV, creatinine level, three-vessel CAD, GRACE score were higher in non-survivors. Hemoglobin, LVEF were lower in non-survivors. There were no significant differences in terms of arterial hypertension, previous MI, previous revascularization, previous heart failure (HF), previous cerebrovascular accident, peripheral artery disease, chronic lung disease (asthma and/or chronic obstructive pulmonary disease), peptic ulcer disease, troponin level, non-obstructive CAD, percutaneous coronary intervention (PCI) rate.
In the structure of in-hospital mortality, majority of patients died from acute HF (75%), followed by myocardial rupture with cardiac tamponade (11.1%) and multiple organ failure (8.3%). Other causes of in-hospital death are presented in Table 2.
In univariate analysis, age, female gender, ST-elevation, diabetes mellitus, atrial fibrillation, Killip class, hemoglobin at admission, estimated glomerular filtration rate (eGFR), LVEF and three-vessel CAD were associated with in-hospital mortality (all p-value < 0.05) (Table 3). The cut-off for LVEF in ROC curve analysis, was 36% (AUC = 0.692; 0.5548–0.836, p=0.004) for in-hospital mortality.
In multivariate analysis, age (OR 1.07, 95% CI 1.01-1.14, p=0.027), Killip class (OR 2.95, 95%CI 1.67-5.23, p<0.001), hemoglobin at admission (OR 0.97, 95% CI 0.95-0.99, p=0.006), and LVEF ≤36% (OR 8.87, 95% CI 2.95-26.69, p<0.001) were independently associated with the primary endpoint.
Based on the estimated variables in the multivariate model, a nomogram was developed to predict the risk of in-hospital death in patients with MI (Fig. 1). The risk of in-hospital death in MI patients was assessed according to the following equation:
Risk = 1/(1 + e-Z), where Z = -6.683 + 0.083 x Age — 0.028 x Hemoglobin at admission + 1.065 x Killip class + 2.377 x LVEF ≤36%.
A total score was generated by using the number of the points of each factor on the corresponding axis with drawing a vertical line to "Points" axis. Summary the points of all the factors and drawing a vertical line to the "Risk of in-hospital mortality" line to determine the individual’s probability of death during hospitalization.
In ROC curves analysis of a developed nomogram for predicting in-hospital mortality, the AUC was 0.949 (95% CI: 0.925-0.972, p<0.001). The sensitivity and specificity were 91.3% and 89.9%, respectively (Fig. 2). A nomogram showed good calibration (chi-square 3.064, p for Hosmer-Lemeshow test 0.93)
Table 1. The baseline characteristics of the patients
Variables | All patients | Alive (n=676) | Dead (n=36) | p-value |
Age, years, Me (IQR) | 65 (56;74) | 64 (55;73) | 77.5 (67;84) | <0.001 |
Females, n (٪) | 278 (39) | 257 (38) | 21 (58.3) | 0.021 |
ST-elevation, n (٪) | 340 (47.8) | 315 (46.6) | 25 (69.4) | 0.01 |
Arterial hypertension, n (٪) | 634 (89) | 601 (88.9) | 33 (91.7) | 0.787 |
Symptoms of CAD, n (٪) | 328 (46.1) | 298 (44.1) | 30 (83.3) | <0.001 |
Previous MI, n (٪) | 155 (21.8) | 143 (21.2) | 12 (33.3) | 0.097 |
Previous revascularization, n (٪) | 85 (11.9) | 82 (12.1) | 3 (8.3) | 0.790 |
Previous HF, n (٪) | 57 (8.0) | 53 (7.8) | 4 (11.1) | 0.521 |
Diabetes mellitus, n (٪) | 150 (21.1) | 137 (20.3) | 13 (36.1) | 0.034 |
Previous CVA, n (٪) | 51 (7.2) | 46 (6.8) | 5 (13.9) | 0.171 |
Atrial fibrillation, n (٪) | 73 (10.3) | 65 (9.6) | 8 (22.2) | 0.024 |
CKD, n (٪) | 61 (8.6) | 50 (7.4) | 11 (30.6) | <0.001 |
PAD, n (٪) | 26 (3.7) | 26 (3.8) | 0 (0) | 0.636 |
Chronic lung disease, n (٪) | 115 (16.2) | 103 (15.2) | 12 (33.3) | 0.009 |
Peptic ulcer disease, n (٪) | 65 (9.1) | 63 (9.3) | 2 (5.6) | 0.764 |
Charlson comorbidity index, points, Me (IQR) | 4 (3; 6) | 4 (3; 5) | 7 (6; 8.75) | <0.001 |
Anemia, n (٪) | 189 (26.5) | 167 (24.7) | 22(61.1) | <0.001 |
Clinical findings: | ||||
Chest pain, n (٪) | 658 (92.4) | 628 (92.9) | 30 (83.3) | 0.047 |
Dyspnea, n (٪) | 124 (17.4) | 110 (16.3) | 14 (38.9) | 0.002 |
Killip class II-IV, n (٪) | 160 (22.5) | 129 (19.1) | 31 (86.1) | <0.001 |
Troponin I, ng/mL, Me (IQR) | 0.39 (0.09; 2.85) | 0.39 (0.09; 2.74) | 1.14 (0.18; 6.22) | 0.191 |
Hemoglobin, g/L, Me (IQR) | 136 (123; 147) | 137 (124; 147) | 118.5 (105.5; 134) | <0.001 |
Creatinine, µmol/L, Me (IQR) | 94 (80; 107) | 92 (76; 106.7) | 106 (92; 160) | 0.014 |
eGFR, ml/min/1.73 m2, Me (IQR) | 67 (52; 83) | 68 (54,2; 83) | 44 (35; 60) | <0.001 |
LVEF, ٪, Me (IQR) | 45 (40; 54) | 45 (40; 54) | 36 (25; 45) | <0.001 |
Coronary stenosis: | ||||
No lesions/Stenosis <50٪, n (٪) | 73 (10.3) | 72 (10.7) | 1 (2.8) | 0.163 |
Three-vessel CAD, n (٪) | 390 (54.8) | 361 (53.4) | 29 (80.6) | 0.002 |
PCI, n (٪) | 566 (79.5) | 538 (79.6) | 28 (77.8) | 0.832 |
GRACE score, points, Me (IQR) | 117 (98; 141) | 115 (97; 137) | 167 (148.2; 193) | <0.001 |
CAD — coronary artery disease, CKD — chronic kidney disease, CVA — cerebrovascular accident, eGFR — estimated glomerular filtration rate, GRACE — Global Registry of Acute Coronary Events, HF — heart failure, IQR — interquartile range, LVEF — left ventricular ejection fraction, | ||||
Table 2. Etiology for in-hospital mortality in patients with acute myocardial infarction
Cause | Absolute value, n | Frequency, % |
Acute heart failure | 27 | 75 |
Myocardial rupture with cardiac tamponade | 4 | 11.1 |
Multiple-organ failure | 3 | 8.3 |
Acute respiratory failure | 1 | 2.8 |
Acute kidney failure | 1 | 2.8 |
Pulmonary embolism | 1 | 2.8 |
Gastrointestinal bleeding | 1 | 2.8 |
Table 3. Univariate and multivariate analysis for the risk factors in predicting the in-hospital mortality
Variable | Univariate Analysis | Multivariate Analysis | ||
OR (95٪ CI) | p-value | OR (95٪ CI) | p-value | |
Age, per year | 1.09 (1.06-1.13) | <0.001 | 1.07 (1.01-1.14) | 0.027 |
Sex, female | 2.28 (1.16-4.51) | 0.017 | 1.46 (0.44-4.91) | 0.683 |
ST elevation | 2.60 (1.26-5.38) | 0.01 | 1.89 (0.66-5.40) | 0.236 |
Diabetes mellitus | 2.22 (1.10-4.50) | 0.026 | 1.25 (0.43-3.69) | 0.681 |
Atrial fibrillation | 2.69 (1.17-6.14) | 0.019 | 2.67 (0.86-8.32) | 0.09 |
Killip class, per class | 5.0 (3.41-7.23) | <0.001 | 2.95 (1.67-5.23) | <0.001 |
Hemoglobin, per g/L | 0.97 (0.95-0.98) | <0.001 | 0.97 (0.95-0.99) | 0.006 |
eGFR ≤60 ml/min/1.73 м2 | 6.21 (2.78-13.89) | <0.001 | 0.82 (0.22-3.03) | 0.768 |
LVEF ≤36% | 13.12 (5.48-31.41) | <0.001 | 8.87 (2.95-26.69) | <0.001 |
Three-vessel CAD | 3.61 (1.56-8.37) | <0.001 | 1.41 (0.46-4.30) | 0.551 |
CAD — coronary artery disease, CI — confidence intervals, LVEF — left ventricular ejection fraction, OR — odds ratio | ||||
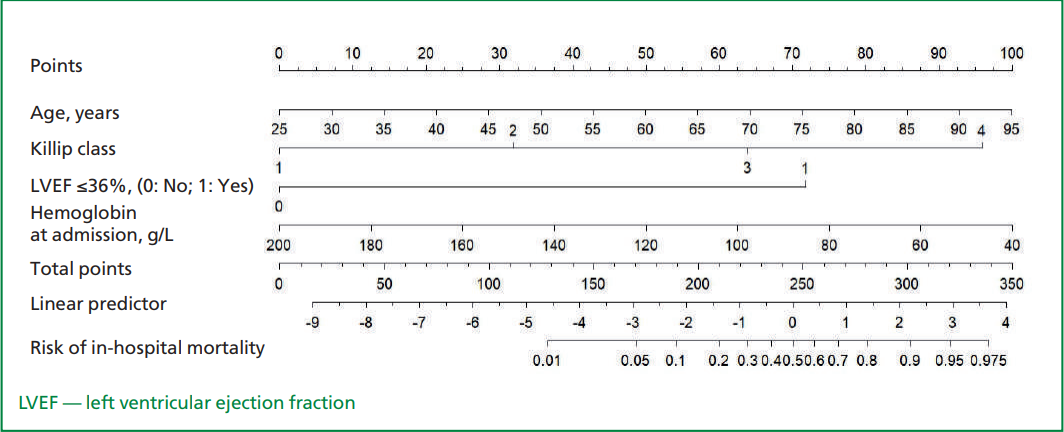
Figure. 1. Nomogram for prediction of in-hospital mortality in acute myocardial infarction. The nomogram included four variables, including age, Killip class, left ventricular ejection fraction and hemoglobin at admission
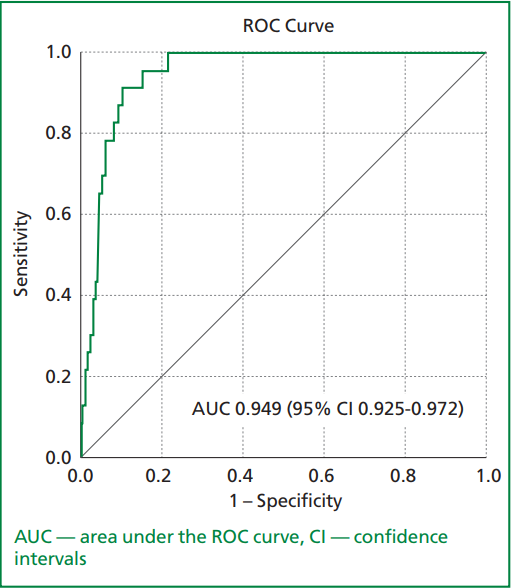
Figure 2. The receiver–operating characteristic (ROC) curve analysis of the nomogram for predicting in-hospital mortality in patients with acute myocardial infarction
Discussion
The present study demonstrated that age, hemoglobin at admission, Killip class and LVEF were independently associated with in-hospital death. Based on results from multivariate analysis, we developed a contemporary risk model to predict in-hospital mortality in patients with acute MI. The model had high predictive potential in predicting in-hospital death.
Age is one of the most important risk factors for a poor outcome in hospitalized patients with acute coronary syndrome (ACS) [15][16]. Median of age among non-survivors in our study was 77.5 years and was an independent factor for in-hospital dearth. Aging causes patients to be physically weaker and frailer. Therefore, old age is an independent predictor of poor prognosis in ACS patients [17]. Additionally, age serves as a prognostic variable in various risk stratification scores for ACS patients [8-10]. In the TIMI III registry, patients over the age of 75 with unstable angina (UA)/non-ST-segment elevation MI (NSTEMI) had more adverse outcomes both in-hospital and within the first six weeks after discharge than those less than 75 years of age [16]. Age and gender are closely associated with in-hospital mortality in acute MI with women being generally older than men at hospitalization for MI. In our study, the risk of in-hospital mortality for women was 2.3 times higher than for men.
The in-hospital mortality rate in our study was 5.06%, which is lower than the 8.8% reported in a recent meta-analysis involving 615,035 MI patients (comprising both ST-segment elevation MI (STEMI) and NSTEMI/UA) [18]. During hospitalization, acute HF emerged as the primary cause of death, accounting for three-fourths of the cases. These results are consistent with those of O. G. Sivkov, who examined 1120 patients with acute MI. The author reported an in-hospital mortality rate of 6.0% (n=67), with acute HF contributing to 70.1% of the deaths (32 cases with acute HF and 15 cases with cardiogenic shock), followed by multiple-organ failure at 28.3% [19].
Our multivariate analysis affirmed the continued relevance of Killip classification and LVEF for effective risk stratification in these patients. Killip classification categorizes patients based on physical examination findings that indicate left ventricular dysfunction and HF [20-22]. According to our results, an increase of in Killip degree was associated with an increased odds ratio of 2.95 for in-hospital mortality. These findings are in the line with an analysis of international data from the GRACE registry involving 3917 NSTEMI patients and 4960 UA patients, where Killip class II or III on admission independently predicted in-hospital death (OR, 2.2; p<0.0001) [23]. A broader analysis of data from 26,090 patients across the GUSTO IIb, PURSUIT, PARAGON A, and PARAGON B trials revealed a significant correlation between Killip class and survival [24]. Patients with Killip class II and III/IV had significantly higher mortality rates at 30 days (3% versus 9% and 14%) and six months (5% versus 15% and 23%) compared to patients with Killip class I. Killip class has proven to be a valuable predictor for adverse outcomes in acute MI patients, incorporated into several risk scores [8-10].
The baseline measurement of LVEF has been identified as the most influential determinant of both in-hospital [25][26] and long-term mortality [27, 28], making its inclusion imperative in risk models for maximal predictive accuracy. In a study by T. Sato et al. [26], involving 1102 acute MI patients undergoing primary PCI, multivariable analysis revealed that LVEF ≤33% independently predicted in-hospital death, with an AUC of 0.79 (p<0.001). Further, incorporation of LVEF into the TIMI score, as opposed to the TIMI risk score alone, resulted in significant enhancements in predicting in-hospital death (AUC: 0.854 vs. 0.803, p=0.033) in a cohort of 673,673 patients with STEMI [27]. Regarding long-term risk stratification, LVEF has proven to be a valuable predictor in various scores. For instance, LVEF was included in the CADILLAC score for predicting one-year mortality after primary PCI for acute AMI, with a cutoff value of LVEF <40% [29]. Consistent with these findings, H. K. Kim et al. developed the new KAMIR score from a cohort of 3,997 hospital-discharged patients with acute MI. The KAMIR score, incorporating six independent variables, including LVEF, demonstrated significant differences in predictive accuracy for one-year mortality compared to the GRACE score (AUC 0.83 vs. 0.76, p=0.0089, respectively). LVEF <40% in this study was associated with a hazard ratio (HR) of 2.24 (95% CI 1.47–3.41) for one-year mortality [30]. In our current study, Killip class at presentation, consistently featured in previous scores remained an independent predictor of reduced in-hospital survival, alongside baseline LVEF. This underscores the significance of clinical examination for signs of mild to moderate HF, even when left ventricular function is preserved.
Anemia is increasingly recognized as a condition strongly predictive of adverse outcomes in patients with acute MI, both in the short term [31-33] and long term [33-35]. In a study by J. J. González-Ferrer et al., involving 542 high-risk ACS patients, anemia was present in 147 patients (27.1%) at admission [32]. The study found that the admission hemoglobin level (OR=1.4 for each 1 g/dL below normal; 95% CI, 1.1-1.8; p=0.003) was independently associated with in-hospital all-cause mortality or cardiogenic shock, after adjusting for other variables. Another study by M. G. Colombo et al. [36] was performed in 2011 consecutively hospitalized acute MI patients. Mild anemia (defined as hemoglobin concentration of 11 to <12 g/dL in women and 11 to <13 g/dL in men) and moderate to severe anemia (defined as hemoglobin concentration of <11 g/dL) were found in 183 (9.1%) and 100 (5%) patients, respectively. The Cox regression analysis showed significantly increased mortality risks in both patients with mild anemia (HR 1.74, 95% CI 1.23–2.45) and moderate to severe anemia (HR 2.05, 95% CI 1.37–3.05) compared to patients without anemia. Anemia represents a potentially modifiable risk factor, and its incremental prognostic value demonstrated in the current analysis highlights the imperative for tailored therapeutic strategies aimed at mitigating its impact and improving overall prognostic outcomes in affected patients.
The study limitations
Our observational study has such limitations as non-randomization and unmeasured confounding factors. For instance, the exclusion of the variable "previous cerebrovascular accident" despite its known association with increased mortality risk in acute MI patients [37] may compromise the comprehensiveness of our findings. Being single centered with a small sample size is another limitation, though the study’s prospective nature adds strength. Thirdly, treatment decisions were left to attending cardiologists, making it difficult to assess the benefits of individualized therapy. At last, the lack of external validation for the nomogram in another acute MI patient cohort limits the generalizability of our findings and reduces the model’s clinical implementation. External validation is necessary to confirm the nomogram’s effectiveness in real-world scenarios.
Conclusion
Age, Killip class, hemoglobin at admission, and LVEF were identified as factors associated with higher in-hospital mortality in patients with acute MI. The most common etiology of in-hospital mortality was acute HF. The nomogram for predicting in-hospital mortality demonstrated high prognostic potential, allowing for the identification of patients at high-risk of adverse outcome, and targeted therapeutic strategies may be needed to improve the survival of patients with acute MI.
Relationships and Activities. None.
Funding. The study was performed with the support of Peoples’ Friendship University of Russia.
1 World Health Organization. The top 10 causes of death. World Health Organization, 9 December 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
2 World Health Organization. Nutritional anaemias: report of a WHO scientific group [meeting held in Geneva from 13 to 17 March 1967]. World Heal Organ Tech Rep Ser. 1968;405:1-37. Available from: https://iris.who.int/handle/10665/40707.
References
1. Vaduganathan M, Mensah GA, Turco JV, et al. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol. 2022;80(25):2361-71. DOI:10.1016/j.jacc.2022.11.005.
2. Timmis A, Vardas P, Townsend N, et al; Atlas Writing Group, European Society of Cardiology. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. 2022;43(8):716-99. DOI:10.1093/eurheartj/ehab892.
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update A Report From the American Heart Association. Circulation. 2017;135(10):e146-e603. DOI:10.1161/CIR.0000000000000485.
4. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356(23):2388-98. DOI: 10.1056/NEJMsa053935.
5. de Vreede JJ, Gorgels AP, Verstraaten GM, et al. Did prognosis after acute myocardial infarction change during the past 30 years? A meta-analysis. J Am Coll Cardiol. 1991;18(3):698-706. DOI:10.1016/0735-1097(91)90792-8.
6. Peterson ED, Shah BR, Parsons L, et al. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156(6):1045-55. DOI:10.1016/j.ahj.2008.07.028.
7. Kontos MC, Rennyson SL, Chen AY, et al. The association of myocardial infarction process of care measures and in-hospital mortality: A report from the NCDR®. Am Heart J. 2014;168(5):766-75. DOI:10.1016/j.ahj.2014.07.005.
8. Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333(7578):1091. DOI:10.1136/bmj.38985.646481.55.
9. Antman EM, Cohen M, Bernink PJ, et al. The TIMI Risk Score for Unstable Angina/Non–ST Elevation MI. JAMA. 2000;284(7):835-42. DOI:10.1001/jama.284.7.835.
10. Bhatt DL, Roe MT, Peterson ED, et al; CRUSADE Investigators. Utilization of Early Invasive Management Strategies for High-Risk Patients With Non–ST-Segment Elevation Acute Coronary Syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA. 2004;292(17):2096-104. DOI:10.1001/jama.292.17.2096.
11. Thygesen K, Alpert J, Jaffe A, et al; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-35. DOI:10.1161/CIR.0b013e31826e1058.
12. Fox KA, Fitzgerald G, Puymirat E, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open. 2014;4(2):e004425. DOI:10.1136/bmjopen-2013-004425.
13. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233-70. DOI:10.1093/ehjci/jev014.
14. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-45.
15. Stone PH, Thompson B, Anderson H V, et al. Influence of race, sex, and age on management of unstable angina and non-Q-wave myocardial infarction: The TIMI III registry. JAMA. 1996;275(14):1104-12.
16. Goldberg RJ, McCormick D, Gurwitz JH, et al. Age-related trends in short- and long-term survival after acute myocardial infarction: a 20-year population-based perspective (1975-1995). Am J Cardiol. 1998;82(11):1311-7. DOI:10.1016/S0002-9149(98)00633-X.
17. Canivell S, Muller O, Gencer B, et al. Prognosis of cardiovascular and non-cardiovascular multimorbidity after acute coronary syndrome. PLoS One. 2018;13(4):e0195174. DOI:10.1371/journal.pone.0195174.
18. Wu J, Hall M, Dondo TB, et al. Association between time of hospitalization with acute myocardial infarction and in-hospital mortality. Eur Heart J. 2019;40(15):1214-21. DOI:10.1093/eurheartj/ehy835.
19. Sivkov OG. Factors Associated With Hospital Mortality in Acute Myocardial Infarction. Kardiologiia. 2023;63(11):29-35 (In Russ.) DOI:10.18087/cardio.2023.11.n2406.
20. Killip T 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20(4):457-64. DOI:10.1016/0002-9149(67)90023-9.
21. Vicent L, Velásquez-Rodríguez J, Valero-Masa MJ, et al. Predictors of high Killip class after ST segment elevation myocardial infarction in the era of primary reperfusion. Int J Cardiol. 2017;248:46-50. DOI:10.1016/j.ijcard.2017.07.038.
22. Zhang Q, Zhou L, Cai HL, Lu HH. Relationship of the ORBIT and HAS-BLED scores with Killip class 3-4 in patients with ST-segment elevation myocardial infarction. Medicine (Baltimore). 2019;98(8):e14578. DOI:10.1097/MD.0000000000014578.
23. Steg PG, Dabbous OH, Feldman LJ, et al; Global Registry of Acute Coronary Events Investigators. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation. 2004;109(4):494-9. DOI:10.1161/01.CIR.0000109691.16944.DA.
24. Khot UN, Jia G, Moliterno DJ, et al. Prognostic importance of physical examination for heart failure in non–ST-elevation acute coronary syndromes: the enduring value of Killip classification. JAMA. 2003;290(16):2174-81. DOI:10.1001/jama.290.16.2174.
25. Wichian C, Morasert T, Nilmoje T, Chichareon P. Prevalence and predictors associated with in-hospital mortality in acute ST segment elevation myocardial infarction after reperfusion therapy in developing country. Cardiovasc Diagn Ther. 2020;10(5):1264-69. DOI:10.21037/cdt-20-398.
26. Sato T, Saito Y, Suzuki S, et al. Prognostic Factors of In-Hospital Mortality in Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. Life (Basel). 2022;12(10):1672. DOI:10.3390/life12101672.
27. Wei XB, Liu YH, He PC, et al. Additive prognostic value of left ventricular ejection fraction to the TIMI risk score for in-hospital and long-term mortality in patients with ST segment elevation myocardial infarction. J Thromb Thrombolysis. 2017;43(1):1-6. DOI:10.1007/s11239-016-1407-7.
28. Yoshioka G, Tanaka A, Watanabe N, et al. Prognostic impact of incident left ventricular systolic dysfunction after myocardial infarction. Front Cardiovasc Med. 2022;9:1009691. DOI:10.3389/fcvm.2022.1009691.
29. Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: The CADILLAC risk score. J Am Coll Cardiol. 2005;45(9):1397-405. DOI:10.1016/j.jacc.2005.01.041.
30. Kim HK, Jeong MH, Ahn Y, et al; Other Korea Acute Myocardial Infarction Registry Investigators. Hospital discharge risk score system for the assessment of clinical outcomes in patients with acute myocardial infarction (Korea Acute Myocardial Infarction Registry [KAMIR] Score). Am J Cardiol. 2011;107(7):965-71.e1. DOI:10.1016/j.amjcard.2010.11.018.
31. Salisbury AC, Amin AP, Reid KJ, et al. Hospital-acquired anemia and in-hospital mortality in patients with acute myocardial infarction. Am Heart J. 2011;162(2):300-9.e3. DOI:10.1016/j.ahj.2011.05.021.
32. González-Ferrer JJ, García-Rubira JC, Balcones DV, et al. Influence of hemoglobin level on in-hospital prognosis in patients with acute coronary syndrome. Rev Esp Cardiol. 2008;61(9):945-52. DOI:10.1157/13125516.
33. Younge JO, Nauta ST, Akkerhuis KM, et al. Effect of anemia on short- and long-term outcome in patients hospitalized for acute coronary syndromes. Am J Cardiol. 2012;109(4):506-10. DOI:10.1016/j.amjcard.2011.09.046.
34. Bassand JP, Afzal R, Eikelboom J, et al; OASIS 5 and OASIS 6 Investigators. Relationship between baseline haemoglobin and major bleeding complications in acute coronary syndromes. Eur Heart J. 2010;31(1):50-8. DOI:10.1093/eurheartj/ehp401.
35. Vicente-Ibarra N, Marín F, Pernías-Escrig V, et al. Impact of anemia as risk factor for major bleeding and mortality in patients with acute coronary syndrome. Eur J Intern Med. 2019;61:48-53. DOI:10.1016/j.ejim.2018.12.004.
36. Colombo MG, Kirchberger I, Amann U, et al. Admission serum potassium concentration and long-term mortality in patients with acute myocardial infarction: results from the MONICA/KORA myocardial infarction registry. BMC Cardiovasc Disord. 2017;17(1):198. DOI:10.1186/s12872-017-0635-x.
37. Brammås A, Jakobsson S, Ulvenstam A, Mooe T. Mortality after ischemic stroke in patients with acute myocardial infarction: predictors and trends over time in Sweden. Stroke. 2013;44(11):3050-5. DOI:10.1161/STROKEAHA.113.001434.
About the Authors
T. H. HoangViet Nam
Truong Huy Hoang.
Ho Chi Minh City
V. V. Maiskov
Russian Federation
Victor V. Maiskov.
Moscow
I. A. Merai
Russian Federation
Imad A. Merai.
Moscow
Z. D. Kobalava
Russian Federation
Zhanna D. Kobalava.
Moscow
Supplementary files
Review
For citations:
Hoang T.H., Maiskov V.V., Merai I.A., Kobalava Z.D. Predictors and etiology of in-hospital mortality in patients with acute myocardial infarction. Rational Pharmacotherapy in Cardiology. 2024;20(3):278-284. https://doi.org/10.20996/1819-6446-2024-3003. EDN: NHUKWW
JATS XML
















































