Scroll to:
Non-High Density Lipoprotein Cholesterol: A Modern Benchmark for Assessing Lipid Metabolism Disorders
https://doi.org/10.20996/1819-6446-2022-07-01
Abstract
Aim. To perform a population analysis of Non-High Density Lipoprotein Cholesterol level (non-HDL-c) in Russian population and to evaluate its association with cardiovascular events.
Material and Methods. The material consisted of results obtained from 11 regions of the ESSE-RF1 Study and from 4 regions of the ESSE-RF2 Study. Study protocols were identical. The studies were performed in 2012-2014 and 2017, respectively. Endpoints were assessed in 19041 people aged 35-64 years. The median follow-up was 6.5 years in ESSE RF (1) and 3.8 years in ESSE RF(2). Analysis was performed for three lipid variables: total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and non-HDLC in two samples: the general population sample and the same sample without individuals with coronary heart disease (CHD), myocardial infarction (MI) and/or stroke history and not taking statins (the population sample of "without a history of cardiovascular diseases [CVD]". The analysis of nonlinear associations was performed using the generalized additive Cox model. The combined cardiovascular endpoint was represented by cardiovascular death and nonfatal MI and stroke. Traditional and laboratory FRs, socio-demographic parameters were analyzed. The significance level for all tested hypotheses was set to be 0.05.
Results. The prevalence of elevated non-HDL-C level (>3.7 mmol/l) was found to be 74.6%. No gender differences were found: there was 74.6% for men and 74.5% for women. Both mean values and prevalence of elevated non-HDL-C were increased with age in women, and its level was slightly decreased in men after 55 years old. Almost all analyzed RFs were significantly associated with elevated non-HDL-C in these two population samples. In both samples elevated total CH and elevated LDL-C were associated with all-cause mortality after correction for all RFs. On the contrary, the non-HDL-C was associated with CVD combined end pints. It has been shown that the risk of these end points increases uniformly with increase in levels of non HDL cholesterol, no nonlinear associations were found.
Conclusion. The results of a population-based analysis of non-HDL-C performed in the Russian population for the first time confirmed that elevated non-HDL-C levels contribute significantly to determining the risk of cardiovascular events in the medium term. It can be assumed that the new risk scales (SCORE2 and SCORE OP) proposed by the European Society of Cardiology and the European Society of Preventive Cardiology, which include non-HDL C instead of TC, will allow adequate assessment of 10-year cardiovascular risk for Russians. However, continued monitoring of endpoints in order to obtain stable associations is required.
Keywords
For citations:
Shalnova S.A., Metelskaya V.A., Kutsenko V.A., Yarovaya E.B., Kapustina A.V., Muromtseva G.A., Svinin G.E., Balanova Yu.A., Imaeva A.E., Evstifeeva S.E., Vilkov V.G., Barbarash O.L., Belova O.A., Grinshtein Yu.I., Efanov A.Yu., Kalachikova O.N., Kulakova N.V., Rotar O.P., Trubacheva I.A., Duplyakov D.V., Libis R.A., Viktorova I.A., Redko A.N., Yakushin S.S., Boytsov S.A., Shlyakhto E.V., Drapkina O.M. Non-High Density Lipoprotein Cholesterol: A Modern Benchmark for Assessing Lipid Metabolism Disorders. Rational Pharmacotherapy in Cardiology. 2022;18(4):366-375. https://doi.org/10.20996/1819-6446-2022-07-01
Introduction
The cholesterol theory of atherosclerosis has been leading since the time of N.N. Anichkov and S.S. Khalatov. Numerous studies have established a stable relationship between elevated levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and atherosclerotic cardiovascular diseases (ACVD), as well as mortality [1-3]. Thus, lipid-lowering therapy is considered an important strategy in the primary and secondary CVD prevention. [4-6].
For many years, an elevated LDL-C level has been considered as a major risk factor for CVD, and the success of drug therapy was due to the achievement of its target levels, determined depending on the patient's cardiovascular risk [7][8]. The work of P.H. Frost and R.J. Havel is among the first publications to introduce cholesterol that is non high-density lipoproteins cholesterol (non-HDL-C) as a tool for screening and risk assessment [9]. In 2001, the ATP III (Adult Treatment Panel III) guidelines introduced non-HDL-C as an alternative target for patients with hypertriglyceridemia [10]. And only in recent decades, a paradigm shift has taken place: the level of non-HDL-C was proposed to be considered as the main risk factor for CVD. It was found that the concentration of non-HDL-C is associated with the characteristics of the metabolic syndrome [11], and its high level correlates with the severity of coronary heart disease (CHD), especially in the presence of hypertriglyceridemia [12][13]. The question of the relationship between the level of non-HDL -C and vascular stiffness is also discussed [14]. A Brazilian study showed that the new parameter could be a useful tool for assessing the risk of arterial stiffness in postmenopausal women [14].
Non-HDL-C is the sum of all apolipoprotein (apo) B-containing lipoproteins, and its elevated levels scould be regarded as a «new atherogenic dyslipidemia» [15] and consider as a first-line predictor for detecting dyslipidemia. This position is based on numerous studies comparing non-HDL-C levels with blood levels of LDL-C or apo B [16-18]. The BARI (Bypass Angioplasty Revascularization Investigation) study showed that elevated non-HDL-C level is a significant and independent predictor of non-fatal myocardial infarction (MI) [19]. In 2020, Kathariya G. et al found that non-HDL-C is a much more specific and sensitive parameter for assessing the risk of elevated systolic blood pressure than LDL-C calculated using the Friedwald formula [20].
X. Su et al. and R. Puri et al. reviewed in detail the significance of the benefits of determining the level of non-HDL-C [21][22]. First, there is no additional cost to assessing non-HDL-C, as it can be easily calculated by subtracting the level of HDL-C from the level of TC. Second, TC and HDL-C quantitation methods are standardized, so calculation of nonHDL-C is considered to be a preferred approach than direct determination of LDL-C or apo B levels, because methods for determining apo B have not been rigorously standardized. Third, fasting blood sampling is not required to determine non-HDL-C levels because its level is not affected by diet. Furthermore, the calculation of LDL-C using the Friedwald formula is invalid when triglyceride (TG) levels are >400 mg/dL. Fourth, determination of non-HDL-C levels allows to assess the content of all potentially atherogenic lipoproteins, including very low density lipoproteins cholesterol, and remnants of lipoproteins with a high content of triglycerides. Moreover, the contribution of these lipoproteins to atherogenic risk is taken into account only when determining non-HDL-C, but not LDL-C. And fifthly, LDL-C is currently considered the main target for reducing the risk of АCVD . This also applies to non-HDL-C, because LDL-C is included in the latter as the main component. Finally, an elevated level of non-HDL-C, associated with a high risk of АCVD, is largely due to the presence in the lipid spectrum of highly atherogenic small, dense LDL particles, the accumulation of which is a reflection of an increased level of triglycerides [12][23][24].
It is known that patients remain at significant residual risk of major adverse cardiovascular events when using LDL-C as the primary target of drug therapy despite aggressive treatment. There is also ample evidence to support an association between lower non-HDL-C levels and reduced CVD risk. NonHDL-C is a more integral characteristic of atherogenic particles than LDL-C, and surpasses it in ability to predict major cardiovascular events, so the residual risk becomes significantly lower [12,21]. At present, the significance of non-HDL-C in predicting the risk of (CHD) is well studied: apparently, non-HDL-C is superior to LDL-C in terms of prognostic capabilities [13].
The 2021 guidelines for CVD prevention in clinical practice include non-HDL-C in the definition of total CVD risk instead of TC, highlighting the importance of non-HDL-C in determining CVD prevention strategies and predicting the risk of major complications. Thus, reducing the level of non-HDL-C is defined as one of the main therapeutic goals [25].
Interesting data are presented in the work of K.M. Pencina et al. [26]. This study determined the extent to which non-HDL-C levels in people aged 25-40 years predict future lipid trajectories. Elevated levels of non-HDL-C detected early in life persist in most young adults during later life, leading to a significant increase of population CVD risk . The results obtained suggest that early monitoring of lipid levels in individuals aged under 40 years will identify the majority of people with a high probability of dyslipidemia throughout their life, and therefore with a high longterm risk of developing CVD [26].
At the same time some researchers believe that an elevated level of non-HDL-C is not an optimal indicator of CVD risk, and suggest considering other indicators, for example, the ratio of TC to HDL-C [27].
In Russia, the level of LDL-C is currently used as the leading risk factor for diseases associated with atherosclerosis, and the achievement of target levels for this parameter is the most important indicator of the success of lipid-lowering drugs in the treatment of dyslipidemia.
The aim of this analysis was to study the population characteristics of non-HDL-C and assess its association with cardiovascular events.
Material and methods
The material of the multicenter study «Epidemiology of Cardiovascular Diseases in the Regions of the Russian Federation (ESSE-RF)» was representative samples of free living male and female population aged 35-64 years [median age was 52 (44; 58)] from 11 regions of the Russian Federation. The study was approved by the Independent Ethics Committee of the Federal State Budgetary Institution «National Medical Research Center for Therapy and Preventive Medicine» of the Ministry of Health of Russian Federation? Federal State Budgetary Institution «National Medical Research Center for Cardiology» of the Ministry of Health of Russian Federation, and Federal State Budgetary Institution «National Medical Research Center named after V.A. Almazov» of the Ministry of Health of Russian Federation and co-executing centers. All participants signed an informed consent. Overall study response rate was about 80%. To increase the power of the study, the results of the ESSE-RF2, whose protocol is identical to the protocol of the ESSE-RF1, were included into analysis. The studies were carried out in 2012-2014 and 2017, respectively.
A systematic stratified multi-stage random sample was used, formed according to the territorial principle on the basis of medical institutions.
Sample members were interviewed using a standard questionnaire developed on the basis of adapted international methods. The modular questionnaire contains socio-demographic data (gender, age, education, income level); data on smoking and alcohol consumption status; health data; disease history. This analysis included education, age, place of residence (urban/rural) and family income level.
In addition, blood pressure levels, heart rate (HR), anthropometric parameters, and the biochemical laboratory tests were measured. The following risk factors for CVD: hypertension (blood pressure, BP≥140/90 mmHg or BP <140/90 mmHg with antihypertensive therapy); elevated HRR≥80 beats per minute; TC ≥5.0 mmol/L; LDL-C >3.0 mmol/L; HDL-C<1.0 mmol/L for men and <1.2 mmol/L for women; non-HDL-C >3.7 mmol/L; elevated lipoprotein (a) (Lp(a) >9 mg/dl); hyperuricemia (serum uric acid>400 μmol/l for men and >360 μmol/L for women); hyperglycemia (fasting plasma glucose ≥6.1 mmol/L); increased highly sensitive C-reactive protein ≥3 mg/L; reduced glomerular filtration rate <90 mL/min/1.73 m2; obesity (body mass index ≥30 kg/m2); abdominal obesity (waist circumference ≥102 cm in men and ≥88 cm in women); current smoking.
The prevalence of elevated non-HDL-C was estimated in 22,373 people, including 8,417 men and 13,956 women aged 35-64 years. In a biennial prospective cohort study, endpoints were determined using direct and indirect contact. We first established the participant's vital status, then the causes of death and new non-fatal CVD cases. We obtained mortality data from a regional register with ICD-10 coded causes of death. Morbidity was checked and specified according to the case histories and in the Compulsory Medical Insurance Fund.
The median follow-up was 6.5 (5.3; 6.8) years in ESSE-RF1 and 3.8 (3.6; 3.9) years in ESSE-RF2. A total of 465 all-causes deaths were included in the analysis, 174 out of them were deaths from CVD. The combined endpoint (CEP) was CVD death plus non-fatal MI and stroke. A total of 608 CEPs were registered. 19,041 people were included in the survival analysis.
Statistical analysis was carried out using the R3.6.1 environment. Pearson's nonparametric skewness coefficient was used to estimate the deviation of the distribution from the normal one. It was calculated as the difference between the mean and median, normalized to the standard deviation. Mean and standard deviation (M±SD) were given for unimodal parameters with non-parametric skewness <0.2, median and interquartile range [Me (Q25; Q75)] were given for other quantitative parameters. Qualitative indicators were described by relative frequencies in percent.
Differences between two independent samples were assessed using the Mann-Whitney's test for continuous parameters, and Fisher's exact test for discrete parameters. Linear regression models were used to «adjust» for covariates. Region indicators were introduced into all regression models as covariates to exclude the effect of regions. The Kaplan-Meier's method was used to construct survival curves. Survival curves were compared by log-rank test with Holm's correction for multiple comparisons. The contribution of risk factors to mortality was assessed using univariate and multivariate Cox proportional hazards models.
The analysis was conducted in two samples: the total sample from the ESSE-RF1 and ESSE-RF2 studies, and the same sample excluding individuals with CHD, a history of MI and stroke, or those taking statins («sample without a history of CVD»).
The analysis of non-linear associations was carried out using the generalized additive Cox model. The significance level for all tested hypotheses was taken equal to 0.05.
Results
Table 1 shows age and gender indicators of nonHDL-C in the total sample of ESSE-RF1 and ESSERF2 studies.
Table 1. Distribution of non-high-density lipoprotein cholesterol and the prevalence of its elevated levels in the total sample of ESSE-RF 1 and ESSE-RF2 Studies
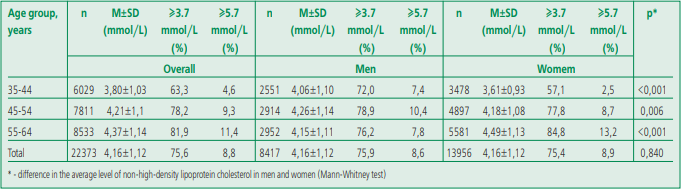
The median level of non-HDL-C doesn't differ significantly from the mean level, that is, the distribution of non-HDL-C levels is close to normal. In the Russian population, a high prevalence of elevated levels of non-HDL-C was found (≥3.7 mmol/L) – 74.6%. No gender differences were found: 74.6% for men and 74.5% for women. The mean values and prevalence of elevated levels of non-HDL-C increase in a gradient in women with age; a slight decrease in this
parameter was noted only in men over 55 years of age. Notably, the prevalence of elevated non-HDL-C in men is higher at younger ages than in women, and conversely, the prevalence of elevated non-HDLC in older women is higher than in men. In other words, the steepness of the age-related rise in the level of non-HDL-C in women was more pronounced.
Table 2 shows associations between social and demographic variables, risk factors, and non-HDL-C levels, demonstrating how age-adjusted non-HDLC levels change in the presence of one or another risk factor. The data in the table show how much non-HDL-C will increase (in mmol/L; age-adjusted) with each of the risk factors. For example, in the general population, the presence of obesity (body mass index ≥30 kg/m2) in men was associated with an increase in non-HDL-C by 0.058 mmol/L (p<0.001); hypertension will lead to a significant increase in the level of non-HDL-C by 0.047 and 0.044 mmol/L in men and women, and in a sample without a history of CVD, hypertension will lead to a significant increase in the level of non-HDL-C by 0.060 and 0.046 mmol/L, respectively. The greatest increase in the level of non-HDL-C in both men and women was noted in the presence of hyperuricemia. Close, although somewhat more pronounced, associations between the level of non-HDL-C and risk factors were obtained in the sample without a history of CVD. Gender differences were no more than 0.01 mmol/L excepting smoking, while associations were found only in women. It's noteworthy that in the population (general) sample, the presence of a history of CVD was significantly associated with a decrease in non-HDL-C level in men, but not in women.
Table 2. Average increase of non high density lipoprotein cholesterol levels under risk factor (in mmol/l, according to the ESSE-RF1 and ESSE-RF2 Studies)
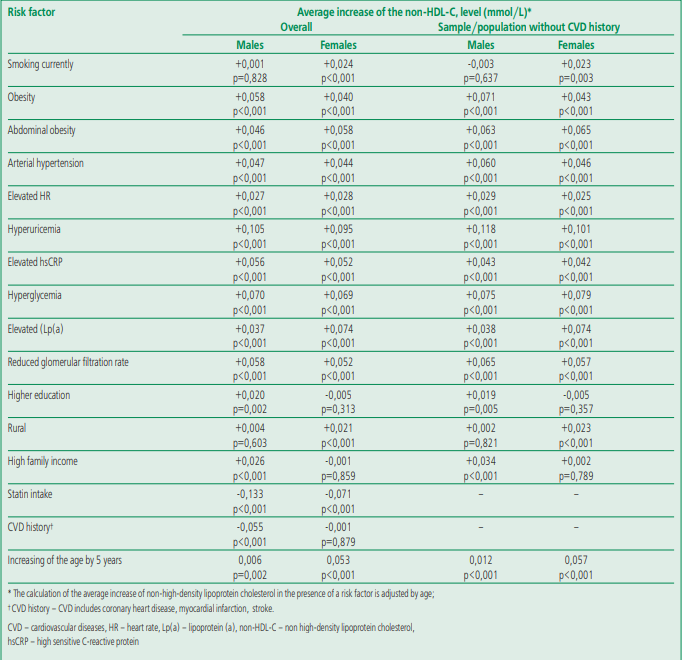
Thus, almost all included into analysis risk factors were significantly associated with elevated levels of non-HDL-C, both in the general population and in the sample without a CVD and taking lipid-lowering drugs (Table 2). Under these conditions, it was important to analyze the relationship of non-HDL-C levels, as well as LDL-C and TC levels, with mortality and CEP.
The level of TC was negatively associated in a multivariate model with total mortality in the general sample and in the sample without a history of CVD. We didn't find significant associations between TC and CVD mortality in the general sample, while associations with CEP were found in the sample without a history of CVD. An elevated level of LDL-C only affects overall mortality in both samples and only negatively. Elevated levels of non-HDL-C increase CVD mortality in general population, and the frequency of CEP increased significantly in both samples. Figure 1 presents the association estimates for the studied lipids and all endpoints in both samples, adjusted for gender, age, body mass index, smoking, statins, and history of CVD. An increase in the level of TC by 1 mmol/L in the sample without a history of CVD statistically significantly increased the CEP by 10% (p=0.04). In the general sample, no relationship was found between the level of TC and CEP. In both samples, there was no relationship between the level of LDL-C and CEP, while associations between nonHDL-C and CEP were statistically significant.
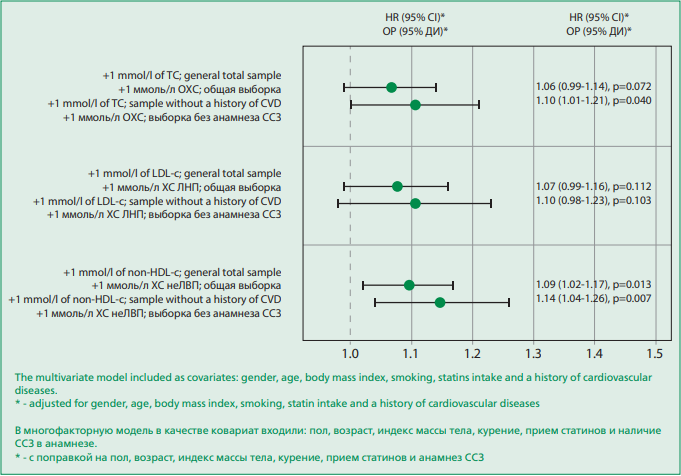
Figure 1. Hazard ratios of total cholesterol, low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol for a combined endpoint in a multivariate model in a total sample and a sample without a history of cardiovascular diseases
Discussion
The present study showed that the distribution of the sample from Russian regions by the level of nonHDL-C appeared to be close to the Gaussian curve, and practically didn't differ from it in similar studies [26, 28]. Mean values of non-HDL-C and the prevalence of elevated its levels didn't differ significantly between men (75.9%) and women (75.4%). We compared our results with data from other studies and found that prevalence in our study was lower than in Northern Sweden and higher than in the Greek ATTICA study, which were conducted around the same years as ESSE-RF. The intake of lipid-lowering drugs was approximately the same in these studies, but the level of non-HDL-C didn't always depend on the proportion of people taking lipid-lowering therapy; 4.4% participants in the ESSE-RF 1 and 6.6% in the ESSE-RF were taking statins, and the prevalence of non-HDL-C ≥3.7 mmol/L, determined according the criteria of F.J. Brunner [28] was 57.9% and 50.1%, respectively. In Northern Sweden and Greece (ATTICA), the prevalence of high non-HDL-C was 71.7% and 48.9%, and statin use was 6.7% and 5.7%, respectively [29].
In the present study, we found a direct relationship between the level of non-HDL-C and age in men from 35 to 55 years; further, the level of non-HDL-C decreased slightly. A direct relationship with age was observed in women in the range of 35-64 years. Despite the fact that the prevalence of elevated nonHDL-C level in men and women was the same in the sample as a whole, the age trend in women turned out to be steeper: from 57.1% in the group of 35-44 years old to 84.8% in the group of 55-64 years old (p<0.001) compared with men. R. Zhang et al. conducted a large-scale, cross-sectional study of 49,201 men and 35,084 women to study agerelated trends in LDL-C and non HDL-C, where blood lipid levels change with age in the general population have been analyzed. Trends in non-HDL-C and LDLC levels were plotted by age (from 18 to 85 years, in one-year increments). Positive trends in non-HDL-C levels were identified in women aged 18 to 56 years and negative trends were identified after 57 years of age. An increase in the level of non-HDL-C was observed in men from 18 to 33 years; then the level reached a plateau from 34 to 56 years, and a decrease in non-HDL-C was noted after 57 years, which was confirmed by correcting the effect of confounders [30]. Thus, the ratio between age and the level of non-HDL-C in the sample of men examined in our study turned out to be almost similar to the results of P. Zhang et al. However, these results need further analysis.
Important determinants of health are education and the family prosperity level. Non-HDL-C in men didn't depend on the level of education and family income level; but its lowest level was observed among women with higher education. Similar relationships were obtained in most other studies. Associations with the main risk factors have been described in many publications, and positive associations were shown in most of them, as in the present study [18- 20].
Of considerable interest are the results of comparing the contribution of non-HDL-C, LDL-C, and TC to all-cause and CVD mortality and their contribution to CEP, obtained in this study on the population sample and the sample without a history of CVD. In these samples, the associations of elevated levels of TC and LDL-C with all-cause death adjusted for all risk factors were found. On the contrary, the level of non-HDL-C was associated with CEP. The result of the generalized additive model (not shown) indicates the absence of a non-linear association between the relative risk in the Cox model and the onset of CEP. Thus, the use of the usual linear Cox model is justified and shows all the information about the association of non-HDL-C and CEP. The result obtained will contribute to the study of non-HDL-C as a predictor of high ACVD risk at the population level using the new scale proposed by the European Society of Cardiology and the European Society of Preventive Cardiology and will make it possible to adequately assess the CVD risk in Russia.
Study limitations. The number of endpoints may not be sufficient to conclude that non-HDL-C should be the primary lipid variable in determining ACVD risk.
Conclusion
The results of this population analysis of nonHDL-C level, conducted for the first time in the Russian Federation, generally confirm the data of other studies that the level of non-HDL-C makes a significant contribution to the cardiovascular risk detection. Nevertheless continued observation of the endpoints is required in order to obtain stable associations.
Relationships and Activities. None.
Funding. The ESSE-RF study was carried out within the framework of the state task for 2012-2014 of the Federal State Budgetary Institution «Russian Cardiology Research and Production Complex» of the Ministry of Health and Social Development of Russia, the Federal State Budgetary Institution «State Research Center for Preventive Medicine» of the Ministry of Health and Social Development of Russia and the Federal State Budgetary Institution «Center for Heart, Blood and Endocrinology named after V.A. Almazov» of the Ministry of Health and Social Development of Russia in part 2 under section I: «Epidemiological studies and modeling of the risk of cardiovascular diseases and their complications». The prospective part of the study was carried out as part of the state task for 2020-2022. No. АААА-А20-120013090086- 0 to the Federal State Budgetary Institution National Medical Research Center for Therapy and Preventive Medicine of the Ministry of Health of the Russian Federation, Moscow «Risk factors for chronic NCDs, their significance for predicting the health of the population of various age groups in some regions of the Russian Federation. Evaluation of the impact on morbidity and mortality (population study)».
References
1. Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. 1979;90(1):85-91. DOI:10.7326/0003-4819-90-1-85.
2. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472. DOI:10.1093/eurheartj/ehx144.
3. Shestov DB, Deev AD, Klimov AN, et al. Increased risk of coronary heart disease death in men with low total and low-density lipoprotein cholesterol in the Russian Lipid Research Clinics Prevalence Follow-up Study. Circulation. 1993;88(3):846-53. DOI:10.1161/01.cir.88.3.846.
4. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111-88. DOI:10.1093/eurheartj/ehz455.
5. Feldman HA, Zuber K, Davis J. Dyslipidemia How Low Should We Go? A Review of Current Lipid Guidelines. Physician Assist Clin. 2017;2(4):633-50. DOI:10.1016/j.cpha.2017.06.004.
6. Dembowski E, Davidson MH. A review of lipid management in primary and secondary prevention. J Cardiopulm Rehabil Prev. 2009;29(1):2-12. DOI:10.1097/HCR.0b013e318192754e.
7. Stroes E. Statins and LDL-cholesterol lowering: an overview. Curr Med Res Opin. 2005;21 Suppl 6:S9-16. DOI:10.1185/030079905X59102.
8. Raal FJ, Hovingh GK, Catapano AL. Familial hypercholesterolemia treatments: Guidelines and new therapies. Atherosclerosis. 2018;277:483-92. DOI:10.1016/j.atherosclerosis.2018.06.859.
9. Frost PH, Havel RJ. Rationale for use of non-high-density lipoprotein cholesterol rather than low-density lipoprotein cholesterol as a tool for lipoprotein cholesterol screening and assessment of risk and therapy. Am J Cardiol. 1998;81(4A):26B-31B. DOI:10.1016/s0002-9149(98)00034-4.
10. Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-97. DOI:10.1001/jama.285.19.2486.
11. Wang S, Tu J, Pan Y. Threshold Effects in the Relationship Between Serum Non-High-Density Lipoprotein Cholesterol and Metabolic Syndrome. Diabetes Metab Syndr Obes. 2019;12:2501-06. DOI:10.2147/DMSO.S232343.
12. Enkhmaa B, Prakash N, Berglund L. Non-HDL-C levels and residual cardiovascular risk: Do population-specific precision approaches offer any advantages? Atherosclerosis. 2018;274:230-1. DOI:10.1016/j.atherosclerosis.2018.05.010.
13. Expert Dyslipidemia Panel, Grundy SM. An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia. J Clin Lipidol. 2013;7(6):561-5. DOI:10.1016/j.jacl.2013.10.001.
14. de Oliveira Alvim R, Mourao-Junior CA, Magalhaes GL, et al. Non-HDL cholesterol is a good predictor of the risk of increased arterial stiffness in postmenopausal women in an urban Brazilian population. Clinics (Sao Paulo). 2017;72(2):106-10. DOI:10.6061/clinics/2017(02)07.
15. Grundy SM, Stone NJ, Bailey AL, et al. 2018 HA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-209. DOI:10.1016/j.jacc.2018.11.002.
16. Aggarwal DJ, Kathariya MG, Verma DPK. LDL-C, NON-HDL-C and APO-B for cardiovascular risk assessment: Looking for the ideal marker. Indian Heart J. 2021;73(5):544-8. DOI:10.1016/j.ihj.2021.07.013.
17. Robinson JG. Are you targeting non-high-density lipoprotein cholesterol? J Am Coll Cardiol. 2009;55(1):42-4. DOI:10.1016/j.jacc.2009.07.056.
18. Cui Y, Blumenthal RS, Flaws JA, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161(11):1413-9. DOI:10.1001/arch-inte.161.11.1413.
19. Bittner V, Hardison R, Kelsey SF, et al.; Bypass Angioplasty Revascularization Investigation. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI). Circulation. 2002;106(20):2537-42. DOI:10.1161/01.cir.0000038496.57570.06.
20. Kathariya G, Aggarwal J, Garg S, et al. Is evaluation of non- HDL- C better than calculated LDL-C in CAD patients? MMIMSR experience. Indian Heart J. 2020;72(3):189-91. DOI:10.1016/j.ihj.2020.05.008.
21. Su X, Kong Y, Peng D. Evidence for changing lipid management strategy to focus on non-high density lipoprotein cholesterol. Lipids Health Dis. 2019;18(1):134. DOI:10.1186/s12944-019-1080-x.
22. Puri R, Mehta V, Lyenger S., et al. Non-HDL Cholesterol and Atherosclerotic Cardiovascular Disease. J Assoc Physicians India. 2020;68(11[Special]):54-58.
23. Orringer CE. Non-HDL cholesterol, ApoB and LDL particle concentration in coronary heart disease risk prediction and treatment. Clin Lipidology. 2013;8(1):69-79. DOI:10.2217/clp.12.89.
24. Maki KC, Bays HE, Dicklin MR. Treatment options for the management of hypertriglyceridemia: Strategies based on the best-available evidence. J Clin Lipidol. 2012;6(5):413-26. DOI:10.1016/j.jacl.2012.04.003.
25. Visseren FLJ, Mach F, Smulders YM, et al.; ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-337. DOI:10.1093/eurheartj/ehab484.
26. Pencina KM, Thanassoulis G, Wilkins JT, et al. Trajectories of Non-HDL Cholesterol Across Midlife: Implications for Cardiovascular Prevention. J Am Coll Cardiol. 2019;74(1):70-9. DOI:10.1016/j.jacc.2019.04.047.
27. Feeman WE. Concerns about the use of non-high-density lipoprotein cholesterol as a lipid predictor. Eur Med J. 2017;2(2):57-64.
28. Brunner FJ, Waldeyer C, Ojeda F, et al. on behalf of the Multinational Cardiovascular Risk Consortium. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet. 2019;394(10215):2173-83. DOI:10.1016/S0140-6736(19)32519-X.
29. Zhang P, Su Q, Ye X, et al. Trends in LDL-C and Non-HDL-C Levels with Age. Aging Dis. 2020;11(5): 1046-1057. DOI:10.14336/AD.2019.1025.
About the Authors
S. A. ShalnovaRussian Federation
Svetlana A. Shalnova.
Moscow.
eLibrary SPIN 9189-8637
V. A. Metelskaya
Russian Federation
Victoria A. Metelskaya.
Moscow.
eLibrary SPIN 2764-8620
V. A. Kutsenko
Russian Federation
Vladimir A. Kutsenko.
Moscow.
eLibrary SPIN 8567-1789
E. B. Yarovaya
Russian Federation
Elena B. Yarovaya.
Moscow.
eLibrary SPIN 5591-8439
A. V. Kapustina
Russian Federation
Anna V. Kapustina.
Moscow.
eLibrary SPIN 1280-2172
G. A. Muromtseva
Russian Federation
Galina A. Muromtseva.
Moscow.
eLibrary SPIN 9872-8010
G. E. Svinin
Russian Federation
Gleb E. Svinin.
Moscow.
Yu. A. Balanova
Russian Federation
Yulia A. Balanova.
Moscow.
eLibrary SPIN 7417-2194
A. E. Imaeva
Russian Federation
Asiia E. Imaeva.
Moscow.
eLibrary SPIN 7568-9285
S. E. Evstifeeva
Russian Federation
Svetlana E. Evstifeeva.
Moscow.
eLibrary SPIN 3706-2581
V. G. Vilkov
Russian Federation
Vladimir G. Vilkov.
Moscow.
eLibrary SPIN 6104-8613
O. L. Barbarash
Russian Federation
Olga L. Barbarash.
Kemerovo.
eLibrary SPIN 5373-7620
O. A. Belova
Russian Federation
Olga A. Belova.
Ivanovo.
Yu. I. Grinshtein
Russian Federation
Yurii I. Grinshtein.
Krasnoyarsk.
eLibrary SPIN 1219-3804
A. Yu. Efanov
Russian Federation
Alexey Yu. Efanov.
Tyumen.
eLibrary SPIN 6423-3149
O. N. Kalachikova
Russian Federation
Olga N. Kalachikova.
Vologda.
eLibrary SPIN 4087-8120
N. V. Kulakova
Russian Federation
Natalia V. Kulakova.
Vladivostok.
eLibrary SPIN 1837-2650
O. P. Rotar
Russian Federation
Oxana P. Rotar.
Saint-Petersburg.
eLibrary SPIN 2416-5178
I. A. Trubacheva
Russian Federation
Irina A. Trubacheva.
Tomsk.
eLibrary SPIN 8605-2140
D. V. Duplyakov
Russian Federation
Dmitry V. Duplyakov.
Orenburg.
eLibrary SPIN 5665-9578
R. A. Libis
Russian Federation
Roman A. Libis.
Samara.
eLibrary SPIN 8292-0051
I. A. Viktorova
Russian Federation
Inna A. Viktorova.
Omsk.
eLibrary SPIN 5171-5592
A. N. Redko
Russian Federation
Andrey N. Redko.
Krasnodar.
eLibrary SPIN 5517-3692
S. S. Yakushin
Russian Federation
Sergey S. Yakushin.
Ryazan.
eLibrary SPIN 7726-7198
S. A. Boytsov
Russian Federation
Sergey A. Boytsov.
Moscow.
eLibrary SPIN 7961-5520
E. V. Shlyakhto
Russian Federation
Evgeniy V. Shlyakhto.
Saint-Petersburg.
eLibrary SPIN 6679-7621
O. M. Drapkina
Russian Federation
Oxana M. Drapkina.
Moscow.
eLibrary SPIN 4456-1297
Review
For citations:
Shalnova S.A., Metelskaya V.A., Kutsenko V.A., Yarovaya E.B., Kapustina A.V., Muromtseva G.A., Svinin G.E., Balanova Yu.A., Imaeva A.E., Evstifeeva S.E., Vilkov V.G., Barbarash O.L., Belova O.A., Grinshtein Yu.I., Efanov A.Yu., Kalachikova O.N., Kulakova N.V., Rotar O.P., Trubacheva I.A., Duplyakov D.V., Libis R.A., Viktorova I.A., Redko A.N., Yakushin S.S., Boytsov S.A., Shlyakhto E.V., Drapkina O.M. Non-High Density Lipoprotein Cholesterol: A Modern Benchmark for Assessing Lipid Metabolism Disorders. Rational Pharmacotherapy in Cardiology. 2022;18(4):366-375. https://doi.org/10.20996/1819-6446-2022-07-01
















































