Scroll to:
Respiratory Muscle Strength in Patients with Coronary Heart Disease and Different Musculoskeletal Disorders
https://doi.org/10.20996/1819-6446-2022-08-04
Abstract
Aim. To measure respiratory muscle strength (RMS) in patients with coronary heart disease (CHD) and different musculoskeletal disorders (MSD).
Material and methods. Patients were divided in four groups according to the MSD. Group I included 52 (13.4%) patients with sarcopenia, group II included 28 (7.2%) patients with osteopenia, group III included 25 (6.5%) patients with osteosarcopenia, group IV included 282 (72.9%) patients without MSD. All patients underwent the assessment of maximal expiratory (МЕР) and maximal inspiratory mouth pressures (MIP).
Results. The mean RMS values were lower than the normative values, and the strength of the expiratory muscles was 1.25 times lower compared to the inspiratory muscles. Both of these parameters were within the normal range in 191 (49.3%) patients, and lower values were noted in 196 (50.7%). An isolated decrease in MIP was observed in 24.8% of patients, an isolated decrease in МЕР in 6.5%, a combined decrease in MIP and МЕР in 19.4% of patients. Comparative analysis of МЕР and MIP (depending on the MSD) did not demonstrate statistically significant differences. Lower МЕР (76.9%) and MIP (75%) values were noted mainly in the group of patients with sarcopenia. A similar pattern was notes in patients with osteosarcopenia and in patients without MSD. Normative values of RMS were observed in patients with osteopenia. Correlation analysis revealed a unidirectional relationship between RMS and the parameters of muscle function (hand grip strength, muscle area and musculoskeletal index) and a multidirectional relationship between МЕР and BMI (r -0.743, p=0.013), MIP and patient age (r -0.624, p=0.021).
Conclusion. Respiratory muscle weakness was diagnosed in half of the patients with coronary heart disease. There were no statistically significant differences in RMS between patients with MSD and isolated CHD, despite lower values in the group with MSD. Correlation analysis revealed an association between RMS and muscle function.
Keywords
For citations:
Bazdyrev E.D., Terentyeva N.A., Galimova N.A., Krivoshapova K.E., Barbarash O.L. Respiratory Muscle Strength in Patients with Coronary Heart Disease and Different Musculoskeletal Disorders. Rational Pharmacotherapy in Cardiology. 2022;18(4):393-401. https://doi.org/10.20996/1819-6446-2022-08-04
Introduction
Aging is a multifactorial biological process associated with a decrease in the physiological functions of the body, primarily due to the accumulation of cellular damage and leading to chronic diseases, increased susceptibility to complications and, as a result, to disability and death [1]. Currently, we have no doubt that age is a non-modifiable risk factor for the development of oncopathology, cardiovascular diseases, age-related weakness, metabolic dysfunction, sarcopenia, osteoarthritis and osteoporosis, as well as many other chronic diseases [2]. Since the elderly represent the fastest growing segment of the population in most developed countries, there is an increasing need for research among this cohort.
Naturally, the aging process is accompanied by changes in the body, including the musculoskeletal system. For example, in an elderly person, upon reaching the sixth decade of life, there is a gradual decrease in bone mineral density (from 1 to 1.5% per year), muscle mass (about 1% per year) and strength (2.5-3% per year), which predisposes to the risk of developing age-related conditions such as osteopenic syndrome, sarcopenia, and osteosarcopenia [3]. Sarcopenia is a generalized process, so it affects not only the systemic skeletal muscles, but also the respiratory muscles [4].
The state of atrophy and weakness of muscle fibers that occurs in the respiratory muscles along with systemic skeletal muscles during aging is known as respiratory sarcopenia [5-7]. Respiratory sarcopenia causes a decrease in respiratory force [8] and lung function [9], which can negatively affect daily activities and quality of life [8][9]. In addition, decreased respiratory muscle strength may lead to adverse clinical outcomes. For example, a decrease in the strength and mass of the respiratory muscles can lead to a violation of ventilation and, as a result, deterioration in oxygenation and the development of multiple organ dysfunction due to hypoxia. It's widely believed that impaired ventilation may also be associated with a higher risk of cardiovascular disease due to decreased exercise tolerance and increased oxidative stress [4].
Studies aimed at assessing muscle function, including respiratory muscles, are primarily relevant for patients undergoing coronary bypass surgery, one of the effective surgical methods for the treatment of coronary heart disease (CHD). Cardiac surgery is often associated with postoperative respiratory dysfunction resulting in hypoventilation, ineffective cough and hypoxemia leading to the risk of postoperative pulmonary complications such as atelectasis, bronchospasm, pneumonia, respiratory failure, acute respiratory distress syndrome, prolonged mechanical ventilation, dysfunction diaphragms and pleural exfusions [10][11]. Respiratory muscle dysfunction during cardiac surgery is a consequence of many factors [12]. The use of grafts (especially the graft of the internal mammary artery) leads to a decrease in the blood supply to the intercostal muscles, sternotomy leads to pain and changes in the mechanics of the chest; sedation, use of myelorelaxants, and pain promote shallow breathing and hypoventilation; artificial circulation and local cooling to protect the myocardium can cause damage to the phrenic nerve; pleural drains (especially intercostal drains) further reduce lung function [12].
Undoubtedly, rehabilitation is the main method that helps to manage these risks and significantly improves the prognosis [13][14]. According to national guidelines for the rehabilitation of patients after coronary bypass surgery, special attention should be paid not only to cardiorehabilitation, but also to prehabilitation, including general physical activity, training of the respiratory muscles, training in diaphragmatic breathing, etc. [15]. Exercise is one of the key components of cardiorehabilitation as it improves blood circulation and increases muscle oxidative capacity in addition to many other benefits. Over time, the portrait of a patient entering for surgical treatment of cardiovascular pathologies changes: the severity of atherosclerotic lesions of the coronary vessels, the severity of the comorbid background, and the patients’ age increase [16].
Thus, not only the study of the influence of ageassociated diseases and syndromes on the outcomes of cardiac surgery, but also the development of methods for the rehabilitation impact on this category of patients, taking into account age-related features of musculoskeletal function, becomes relevant. Federal State Budgetary Scientific Institution «Research Institute of Complex Problems of Cardiovascular Diseases» is conducting a scientific project to study the pathophysiological features of impaired musculoskeletal fat and cardiovascular remodeling as markers of pathological aging. The main hypothesis is that cardiovascular disease may be responsible for earlier pathological aging. This publication presents the results of a study aimed at assessing the respiratory muscle strength in patients with coronary heart disease and different musculoskeletal disorders.
Materials and methods
The study sequentially included 387 patients with a stable form of coronary heart disease who were admitted to the Research Institute of Complex Problems of Cardiovascular Diseases (Kemerovo) from 2019 to 2020 for planned coronary bypass surgery. The study included patients who met the inclusion/exclusion criteria.
Inclusion criteria: 1) stable CHD; 2) planned coronary artery bypass grafting under cardiopulmonary bypass; 3) consent to participate in the study.
Exclusion criteria: 1) neuromuscular diseases; 2) deformation of the chest and spine (scoliotic disease, lordosis); 3) taking certain medications (glucocorticosteroids, antidepressants, barbiturates, muscle relaxants); 4) surgical intervention on the organs of the chest in history; 5) failure to understand and/or follow the procedures of the study protocol; 6) refusal (withdrawal of consent) from participation in the study.
The study was approved by the local ethics committee of the Research Institute of Complex Problems of Cardiovascular Diseases (protocol No. 12 dated December 27, 2019). All participants signed an informed consent form prior to inclusion in the study.
Verification of sarcopenia was carried out in accordance with the criteria of the EWGSOP (2019) based on the analysis of the SARC-F questionnaire, the determination of the hand grip force and the quantitative assessment of skeletal muscles, taking into account the obtained values of the muscle tissue area and the calculated musculoskeletal index when performing multislice computed tomography [17]. Osteopenic syndrome (osteopenia/osteoporosis) was diagnosed based on the World Health Organization (2008) criteria for postmenopausal women; according to the T-test obtained by dual-energy Xray absorptiometry, for men over 50 years old [18]. Osteosarcopenia was diagnosed when sarcopenia combined with osteopenic syndrome in one patient [19].
Four groups were formed according to the above criteria. The first group included 52 (13.4%) patients with isolated sarcopenia, the second group included 28 (7.2%) patients with isolated osteopenic syndrome (osteopenia/osteoporosis), the third group included 25 (6.5%) patients with osteosarcopenia, and the fourth group included 282 (72.9%) participants with CHD without musculoskeletal disorders.
The respiratory muscle strength was studied three days before coronary artery bypass grafting using an EliteDl-220v body plethysmograph (Medical Graphics Corporation, USA) using Breeze Suite 6.2 software in accordance with the criteria of the American Thoracic and European Respiratory Societies (ATS/ERS) [20]. The method for assessing the respiratory muscle strength is based on the measurement of maximum static pressures at the level of the mouth, which the patient creates with closed airways during maximum inhalation and exhalation, followed by an analysis of maximum expiratory pressure (MEP) and maximum inspiratory pressure (MIP) in the mouth. By measuring MIP, the force of the diaphragm is first assessed; by measuring MEP, the intercostal muscles and muscles of the abdominal wall are mainly assessed [21].
We performed 5-6 maneuvers (at least 3) to determine each parameter when measuring MIP and MEP, with a time interval between maneuvers of at least one minute. The study was terminated when the difference between the three maximum values was less than 20%, and the maximum value of the parameter was included in the final protocol. If the maximum result was obtained during the last attempt, it was not taken into account.
Measurements were taken in a sitting position. We used a nose clip to prevent air leakage and a flanged mouthpiece to ensure minimal air leakage from the mouth during maneuvers. During the expiratory maneuver, the patient's cheeks were recommended to be supported to reduce the contribution of buccal muscle contraction. Patients had to take a maximum breath to determine MIP and exhale, followed by an assessment of MEP with the mouthpiece closed. The patient performed MIP maneuver from the level of residual lung volume (after a full expiration), and MEP maneuver from the level of total lung capacity (after a full inspiration). The duration of each maneuver during the respiratory muscle assessment test was at least 1.5 sec.
In this study, MIP and MEP were assessed from the position of the low level of normal (LLN), given the lack of a unified approach to interpreting the respiratory muscle strength. The LLNs for MIP and MEP were calculated using the reference equations proposed by J.A. Evans and W.A. Whitelaw [22] (Table 1). MIP and MEP were considered below standard values if these parameters were below LLN.
According to the ATS/ERS guidelines [20][23], a MIP of 80 cm water column often indicates the absence of clinically significant respiratory muscle weakness.
Table 1. Calculation equations for determining the lower limit of the MIP and MEP norm

Statistical data analysis was performed using the Statistica 6.1 software package (StatSoft Inc., USA). The nature of the data distribution was assessed using the Shapiro-Wilk's test. The distribution of all quantitative data differed from normal. Qualitative scores were presented as frequencies (n, %) and quantitative scores were presented as central trends and scatter: median (Me) and interquartile range (25%; 75%). Comparison of three or more independent groups was performed using Kruskal-Wallis's rank analysis of variations. A 2×2 cross-tabulation analysis was used to evaluate differences in relative values. Hypotheses were tested using the χ² test (Pearson). Two-tailed Fisher's exact test with Yates correction was used for a small number of observations. Spearman's rank correlation coefficient was used to analyze relationships between traits. Mean differences and correlations were considered statistically significant at p≤0.05.
Results
Clinical and anamnestic characteristics of patients with different musculoskeletal disorders are presented in Table 2.
Table 2. Comparative characteristics of patients with coronary artery disease, depending on the type of musculoskeletal status disorder
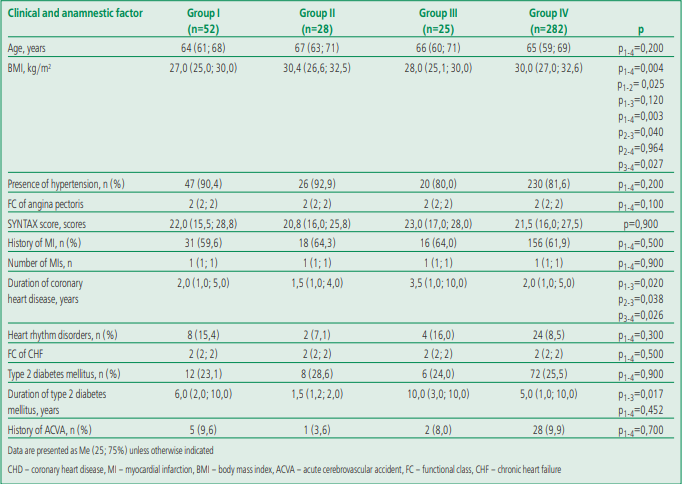
The patients of the analyzed groups had no statistically significant differences in age, violation of the coronary bed (according to the SYNTAX scale) and the main concomitant pathology, with the exception of the duration of coronary heart disease and diabetes mellitus (DM). The duration of coronary heart disease in patients with osteosarcopenia was 2.3 times higher than in patients with osteopenic syndrome and 1.7 times higher in comparison with patients with isolated sarcopenia and persons without musculoskeletal status disorders (MSS).
A similar statistical relationship was noted for the duration of DM. A longer duration of DM has been observed in patients with osteosarcopenia; the shortest duration of DM was registered in the group with isolated osteopenic syndrome. Patients with isolated sarcopenia and without MMS impairment had no significant differences in the duration of DM.
Most of the study participants were overweight or obese, as measured by body mass index (BMI). The highest values of the indicator were registered in patients with isolated osteopenic syndrome (group II) and patients without MMS disorders (group IV). Average BMI values corresponded to obesity of the 1st degree. They were lower in patients with isolated sarcopenia (group I) and osteosarcopenia (group III) and corresponded to overweight.
The respiratory muscle strength in patients with coronary artery disease was below the standard values, and the expiratory respiratory muscle strength [MEP 60 (40; 80) cm of water column] was 1.25 times lower than the inspiratory muscle strength [MIP 75 (42; 92) cm water column], which generally indicates an initially clinically significant weakness of the respiratory muscles in this group of patients. We found that both analyzed parameters (MEP and MIP) corresponded to the norm in 191 (49.3%) patients with coronary heart disease (they were higher than LLN), while various options for reducing the respiratory muscle strength with the predominant option in the form of an isolated decrease in MIP were verified in 196 (50.7%) participants [isolated decrease in MIP, 96 (24.8%), and in MEP, 25 (6.5%); combined decrease in MIP and MEP, 75 (19.4%)]. A comparative analysis of the parameters characterizing the respiratory muscle strength, taking into account the presence/absence of the initial musculoskeletal disorders, as well as an intergroup analysis of patients who initially had different MSS disorders, is presented in Table 3.
Table 3. Comparative characteristics of respiratory muscle strength in patients with stable coronary artery disease
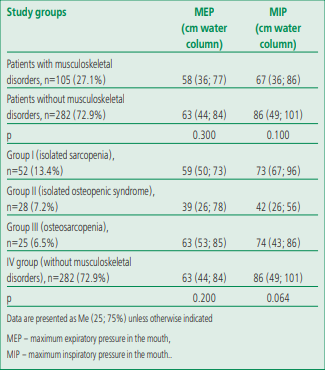
Lower values of MIP (by 1.28 times) and MEP (by 1.08 times) were observed in patients with coronary heart disease and musculoskeletal disorders compared with individuals without them. But the expiratory muscle strength (MEP) was below the normative values even in patients who initially didn't have MSS disorders. But there were no statistically significant differences in the respiratory muscle strength between patients with and without musculoskeletal disorders.
Comparative analysis of MEP and MIP, depending on the variant of musculoskeletal dysfunction, showed regular changes. For example, the lowest strength of the inspiratory and expiratory respiratory muscles was recorded in the group with isolated sarcopenia, the greatest strength was recorded in the group of patients with CHD without MSS disorders, while no statistically significant differences were obtained, except for the trend towards differences in the level of inspiratory muscle strength (p=0.064).
In each group, there were individuals, along with patients, characterized by reduced MEP and MIP parameters, in which the analyzed parameters were within the normative values (Fig. 1). For example, lower values (below LLN) of MIP strength were more common among patients with isolated sarcopenia; accordingly, in this group, the smallest number of patients with the normative value of this parameter was determined. The highest frequency of detection of persons with reduced MEP strength was recorded among persons without MSS disorders and didn't differ from groups of patients who initially had signs of impaired muscle function (isolated sarcopenia, osteosarcopenia). We also note that significantly more often the normative values (above LLN) of the analyzed parameters were observed in patients with osteopenic syndrome.
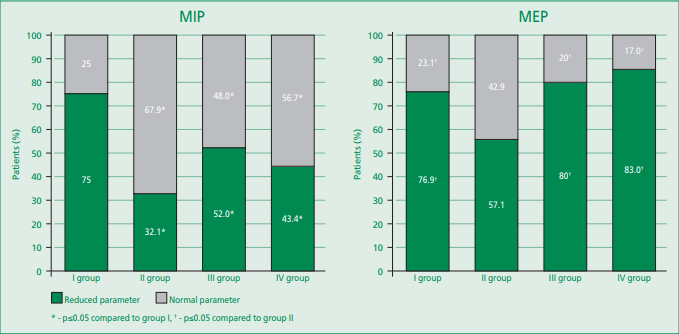
Figure 1. The strength of the respiratory muscles in patients with stable coronary heart disease, depending on the type of violation of the musculoskeletal status
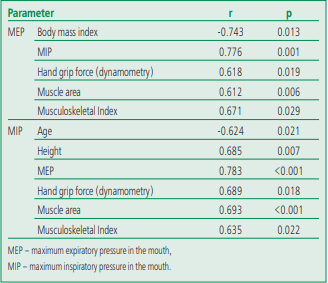
The results of the correlation analysis demonstrated statistically significant relationships (of average strength) between the respiratory muscle strength and the values of a number of clinical parameters (Table 4). For example, the MEP and MIP value had a unidirectional relationship with parameters that characterize muscle function and indicate a muscle status disfunction (hand grip force according to dynamometry, muscle area and musculoskeletal index). In addition, according to the correlation analysis, we revealed a one-sided relationship between MEP and MIP and a multidirectional relationship between MEP and BMI, MIP and the patient's age.
Table 4. Results of correlation analysis of respiratory muscle strength and clinical and instrumental parameters of patients with stable coronary artery disease
Discussion
The results of the presented study allowed us to conclude that clinically significant respiratory muscle weakness was verified in half (50.7%) of patients with coronary heart disease before coronary artery bypass grafting, which is confirmed by MEP and MIP parameters below the normative values. We found a trend towards lower MEP and MIP parameters in the MSS disordered group, but no statistically significant difference. The greatest degree of deviation from the
normative values was determined for the indicator characterizing the expiratory muscle strength (MEP). Probably, the absence of differences can be explained by the fact that 49.3% of the study participants had normative values for the respiratory muscle strength parameters. For example, MEP (23.1%) and MIP (25%) were within normal limits in a quarter of patients, even in the isolated sarcopenia group. The correlation analysis performed demonstrated the relationship between the respiratory muscle strength and other parameters of muscle function (hand grip force, muscle area, musculoskeletal index).
Determining the respiratory muscle strength is currently not a routine study. First of all, it's performed to diagnose diseases of the central and peripheral nervous system, accompanied by weakness of the respiratory muscles. In the last decade, works have begun to appear on aging, which consider not only the isolated weakness of the respiratory muscle strength, but also the simultaneous decrease in respiratory function. As a rule, respiratory muscle sarcopenia is not diagnosed in clinical practice due to the lack of a unified concept and diagnostic criteria. In fact, the pathology is widespread mainly among the elderly. A working group from Japan to study this problem proposed the concept and diagnostic
criteria for various variants of respiratory muscle dysfunction in individuals with sarcopenia. The review by A. Nagano et al. [24] presents a number of definitions and diagnostic criteria for such disorders. For example, the term presbypnea has been proposed for age-related changes, accompanied by a decrease in the mass of the respiratory muscles, the development of respiratory muscle weakness and a decrease in respiratory function. At the same time, the concept of respiratory sarcopenia is proposed to be used in the case of a combination of sarcopenia and a decrease in the mass of the respiratory muscles, followed by low respiratory muscle strength and/or deterioration in respiratory function. Sarcopenic respiratory failure suggests the presence of respiratory failure requiring oxygen therapy with signs of impaired respiratory function due to respiratory sarcopenia, in the absence of clinically significant diseases of the bronchopulmonary system.
The main respiratory muscles include the diaphragm and intercostal muscles, which provide ventilation of the lungs under physiological conditions. A number of studies have shown that the respiratory muscle strength and muscle mass decrease with age, which leads to the development of sarcopenia of the diaphragm muscles [24]. B. Bordoni et al found that transdiaphragmatic pressure and diaphragmatic muscle activity in the elderly are reduced by 20-41% with a decrease in the total respiratory muscle strength by 30% [25]. Another study found histopathological abnormalities in the structure of the diaphragm at autopsy of elderly people, including its small size or altered shape, as well as loss of cytoplasmic integrity [26]. In a study by P.M. Lalley, a decrease in intercostal muscle mass was associated with age [27]. According to a number of studies, the respiratory muscle strength, the thickness of the diaphragm and the respiratory function are impaired in elderly people not only with sarcopenia, but also with senile asthenia syndrome. For example, elderly people with frailty and preasthenia had significantly lower MIP and MEP parameters compared to patients without such signs [8][28].
The results of individual studies prove the relationship between a decrease in the respiratory muscles strength and the clinical manifestations of cardiovascular diseases. For example, P. Verissimo et al [29] showed that respiratory muscle weakness, assessed by the MIP level, is verified in 76% of elderly patients hospitalized for acute heart failure, and respiratory muscle weakness persists even after clinical stabilization of heart failure. Review data by H. Fernández-Rubio et al. [30] support the hypothesis that skeletal muscle deterioration is the main cause of chronic heart failure symptoms. The muscle weakness present in people with chronic heart failure (respiratory muscle weakness is detected in about 50% of patients) occurs more often in the inspiratory muscles than in the muscles of the lower extremities, and is one of the main causes of exercise intolerance. M. Riou et al [31] concluded that skeletal and respiratory muscle weakness contributes significantly to the reduced quality of life and exercise intolerance observed in patients with pulmonary hypertension.
The results of the present study didn't confirm the assumption that the decrease in the respiratory muscle strength is characteristic of all patients with sarcopenia. This fact can be explained by the fact that, firstly, we didn't analyze the respiratory muscle mass and respiratory function, which would allow us to fully diagnose respiratory sarcopenia. Secondly, we diagnosed sarcopenia according to the latest version of the EWGSOP criteria (2019), which differs from previous versions. We also note that respiratory muscle weakness, probably due to the manifestation of heart failure or pulmonary hypertension, was verified even in patients who didn't have signs of a decrease in skeletal muscle mass and function (a group of patients with coronary heart disease without musculoskeletal disorders).
According to the results of our correlation analysis, the respiratory muscle strength has a significant inverse relationship with age, but a direct relationship with the parameters characterizing the muscle status, namely, the hand grip force, muscle area, and musculoskeletal index. The data obtained are consistent with the results previously presented in the literature [32-35]. For example, a study by H. Shin et al showed that inspiratory and expiratory muscle strength was positively correlated with musculoskeletal index (MIP, r=0.451, p<0.05; MEP, r=0.388, p<0.05) and with hand grip force (r=0.560, p<0.01 and r=0.393, p<0.05, respectively). The authors also confirmed this relationship in an age-adjusted regression analysis, demonstrating a correlation of MIP with hand grip force (β=1.876, p<0.001) and musculoskeletal index (β=1.964, p=0.01), but expiratory force muscle (MEP) was associated only with hand grip force (β=1.102, p=0.02) [32]. The authors of a study of 65 Brazilian elderly people demonstrated a positive correlation between lower extremity muscle function and inspiratory muscle strength by measuring knee flexor/extensor muscle strength, walking speed in 6 min, and MIP/MEP [34]. H.J. Ro et al came to a similar conclusion, showing that MIP correlates with knee extensor strength and hand grip force in both men and women, including young adults [35]. According to a study of 62 men living in care homes in Turkey, MIP is significantly associated with hand grip force independently of other factors [33].
Taking into account the comorbid background and age characteristics of patients admitted for planned cardiac surgery, prehabilitation issues are currently given considerable attention due to the higher risks of complications during the hospital period. Apparently, the assessment of the respiratory muscle strength before coronary bypass surgery should be included in the standard of preoperative examination of patients for the preparation of prehabilitation programs. It has been proven that respiratory muscle training can be effective in improving not only overall physical function, but also respiratory function and muscle strength. Respiratory muscle training leads to a decrease in the severity of dyspnea (from the level of the base index of dyspnea), an increase in exercise tolerance, assessed in the 6-minute walk test in patients with chronic obstructive pulmonary disease [36], and has a positive effect on maximum expiratory pressure in adults [37] and increases skeletal muscle strength in elderly people with sarcopenia [38]. A recent meta-analysis demonstrated that respiratory muscle training improves respiratory muscle strength, lung function (increases forced expiratory volume in the first second and forced lung vital capacity), exercise tolerance (increases distance in the 6-minute walk test), and reduces length of stay in the hospital after heart surgery [12]. In addition, according to the results of a recent study of patients with COVID-19, the use of the method of the respiratory muscle training contributed to the improvement of the speed and volume indicators of the external respiration function and the diffusion capacity of the lungs [39]. Also, some scientists have demonstrated that the loss of body weight and respiratory muscle function can be prevented using a program of the inspiratory and expiratory muscle training, which ultimately leads to an increase in the respiratory muscle strength in older people with a sedentary lifestyle [32].
Study limitations. A limitation of this study is the small number of included patients with different musculoskeletal disorders, which may affect the power of the results.
Conclusion
Half of patients with coronary heart disease before coronary bypass surgery were diagnosed with the clinically significant respiratory muscle weakness, assessed by measuring MEP and MIP. The lowest values are typical for the expiratory muscle strength (MEP), which indicates the dysfunction of the predominantly intercostal muscles and the muscles of the abdominal wall in comparison with the muscles of the diaphragm. In the study, we did not find statistically significant differences in respiratory muscle strength between patients with musculoskeletal disorders (including various variants) and isolated CHD, despite lower median MEP and MIP in the musculoskeletal disorders group. Correlation analysis data confirmed the relationship between respiratory muscle strength and parameters characterizing muscle function. The obtained results demonstrate the need for a test to assess the respiratory muscle strength in patients before coronary bypass surgery for the subsequent personalized approach to pre- and rehabilitation.
Relationships and Activities. None.
Financing. The study was funded by the Russian Science Foundation (grant number 22-15-00305 “Pathophysiological features of osteosarcopenic obesity in patients with multifocal atherosclerosis as a marker of biological aging”).
References
1. Fraser HC, Kuan V, Johnen R, et al. Biological mechanisms of aging predict age-related dis-ease cooccurrence in patients. Aging Cell. 2022;21(4):e13524. DOI:10.1111/acel.13524.
2. Farr JN, Almeida M. The Spectrum of Fundamental Basic Science Discoveries Contributing to Organismal Aging. J Bone Miner Res. 2018;33(9):1568-84. DOI:10.1002/jbmr.3564.
3. Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11(3):609-18. DOI:10.1002/jcsm.12567.
4. Vilaro J, Ramirez-Sarmiento A, Juana MA, et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med. 2010;104(12):1896-902. DOI:10.1016/j.rmed.2010.05.001.
5. Kera T, Kawai H, Hirano H, et al. Definition of Respiratory Sarcopenia With Peak Expiratory Flow Rate. J Am Med Dir Assoc. 2019;20(8):1021-5. DOI:10.1016/j.jamda.2018.12.013.
6. Vang P, Vasdev A, Zhan WZ, et al. Diaphragm muscle sarcopenia into very old age in mice. Physiol Rep. 2020;8(1):e14305. DOI:10.14814/phy2.14305.
7. Greising SM, Mantilla CB, Gorman BA, et al. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013;48(9):881-7. DOI:10.1016/j.exger.2013.06.001.
8. Ohara DG, Pegorari MS, Oliveira Dos Santos NL, et al. Respiratory Muscle Strength as a Discriminator of Sarcopenia in Community-Dwelling Elderly: A Cross-Sectional Study. J Nutr Health Aging. 2018;22(8):952-8. DOI:10.1007/s12603-018-1079-4.
9. Greising SM, Mantilla CB, Medina-Martmez JS, et al. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am J Physiol Lung Cell Mol Physiol. 2015;309(1):L46-52. DOI:10.1152/ajplung.00064.2015.
10. Shakouri SK, Salekzamani Y, Taghizadieh A, et al. Effect of respiratory rehabilitation before open cardiac surgery on respiratory function: a randomized clinical trial. J Cardiovasc Thorac Res. 2015;7(1):13-7. DOI:10.15171/jcvtr.2015.03.
11. Chen X, Hou L, Zhang Y, et al. The effects of five days of intensive preoperative inspiratory muscle training on postoperative complications and outcome in patients having cardiac surgery: a randomized controlled trial. Clin Rehabil. 2019;33(5):913-22. DOI:10.1177/0269215519828212.
12. Dsouza FV, Amaravadi SK, Samuel SR, et al. Effectiveness of Inspiratory Muscle Training on Respiratory Muscle Strength in Patients Undergoing Cardiac Surgeries: A Systematic Review With Meta-Analysis. Ann Rehabil Med. 2021;45(4):264-73. DOI:10.5535/arm.21027.
13. Moreira JMA, Grilo EN. Quality of life after coronary artery bypass graft surgery - results of cardiac rehabilitation programme. J Exerc Rehabil. 2019;15(5):715-22. DOI:10.12965/jer.1938444.222.
14. Osailan A, Abdelbasset WK. Exercise-based cardiac rehabilitation for postcoronary artery by-pass grafting and its effect on hemodynamic responses and functional capacity evaluated using the Incremental Shuttle Walking Test: A retrospective pilot analysis. J Saudi Heart Assoc. 2020;32(1):25-33. DOI:10.37616/2212-5043.1005.
15. Russian clinical guidelines. Coronary artery bypass grafting in patients with is-chemic heart disease: rehabilitation and secondary prevention. Cardiosomatics. 2016;7(3-4):5-71 (In Russ.) DOI:10.26442/CS45210.
16. Ivanov SV, Sumin AN. Current trends in routine myocardial revascularization. Complex Is-sues of Cardiovascular Diseases. 2021;10(2):25-35 (In Russ.) DOI:10.17802/2306-1278-2021-10-2-25-35.
17. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31. DOI:10.1093/ageing/afy169.
18. Mel'nichenko GA, Belaya ZE, Rozhinskaya LY, et al. Russian federal clinical guidelines on the diagnostics, treatment, and prevention of osteoporosis. Problems of Endocrinology. 2017;63(6):392-426 (In Russ.) DOI:10.14341/probl2017636392-426.
19. Bazdyrev ED, Terentyeva NA, Krivoshapova KE, et al. Prevalence of Musculoskeletal Disorders in Patients with Coronary Artery Disease. Rational Pharmacotherapy in Cardiology. 2021;17(3):369-75 (In Russ.) DOI:10.20996/1819-6446-2021-06-03.
20. American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518-624. DOI:10.1164/rccm.166.4.518.
21. Mora Romero UJ, Gochicoa Rangel L, Guerrero Zúñiga S, et al. Maximal inspiratory and expiratory pressures: Recommendations and procedure. Neumol Cir Torax. 2019;78(Supl.2):S135-S141. (in Spanish) DOI:10.35366/NTS192F.
22. Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54(10):1348-59.
23. Laveneziana P, Albuquerque A, Aliverti A, et al. ERS Statement on Respiratory Muscle Testing at Rest and during Exercise. Eur Respir J. 2019;53(6):1-34. DOI:10.1183/13993003.01214-2018.
24. Nagano A, Wakabayashi H, Maeda K, et al. Respiratory Sarcopenia and Sarcopenic Respiratory Disability: Concepts, Diagnosis, and Treatment. J Nutr Health Aging. 2021;25(4):507-15. DOI:10.1007/s12603-021-1587-5.
25. Bordoni B, Morabito B, Simonelli M. Ageing of the Diaphragm Muscle. Cureus. 2020;12(1):e6645. DOI:10.7759/cureus.6645.
26. Nucci RAB, de Souza RR, Suemoto CK, et al. Diaphragm muscle structure in the elderly: Findings from an autopsy study. Acta Histochem. 2020;122(2):151487. DOI:10.1016/j.acthis.2019.151487.
27. Lalley PM. The aging respiratory system--pulmonary structure, function and neural control. Respir Physiol Neurobiol. 2013;187(3):199-210. DOI:10.1016/j.resp.2013.03.012.
28. Kera T, Kawai H, Hirano H, et al. Relationships among peak expiratory flow rate, body composition, physical function, and sarcopenia in community-dwelling older adults. Aging Clin Exp Res. 2018;30(4):331-40. DOI:10.1007/s40520-017-0777-9.
29. Verissimo P, Timenetsky KT, Casalaspo TJA, et al. High Prevalence of Respiratory Muscle Weakness in Hospitalized Acute Heart Failure Elderly Patients. PLoS One. 2015;10(2):e0118218. DOI:10.1371/journal.pone.0118218.
30. Fernández-Rubio H, Becerro-de-Bengoa-Vallejo R, Rodríguez-Sanz D, et al. Unraveling the Role of Respiratory Muscle Metaboloreceptors under Inspiratory Training in Patients with Heart Failure. Int J Environ Res Public Health. 2021;18(4):1697. DOI:10.3390/ijerph18041697.
31. Riou M, Pizzimenti M, Enache I, et al. Skeletal and Respiratory Muscle Dysfunctions in Pulmonary Arterial Hypertension. J Clin Med. 2020;9(2):410. DOI:10.3390/jcm9020410.
32. Shin HI, Kim DK, Seo KM, et al. Relation Between Respiratory Muscle Strength and Skeletal Muscle Mass and Hand Grip Strength in the Healthy Elderly. Ann Rehabil Med. 2017;41(4):686-92. DOI:10.5535/arm.2017.41.4.686.
33. Bahat G, Tufan A, Ozkaya H, et al. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. Aging Male. 2014;17(3):136-40. DOI:10.3109/13685538.2014.936001.
34. Giua R, Pedone C, Scarlata S, et al. Relationship between respiratory muscle strength and physical performance in elderly hospitalized patients. Rejuvenation Res. 2014;17(4):366-71. DOI:10.1089/rej.2014.1549.
35. Ro HJ, Kim DK, Lee SY, et al. Relationship Between Respiratory Muscle Strength and Conventional Sarcopenic Indices in Young Adults: A Preliminary Study. Ann Rehabil Med. 2015;39(6):880-7. DOI:10.5535/arm.2015.39.6.880.
36. Beaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin Respir J. 2018;12(7):2178-88. DOI:10.1111/crj.12905.
37. Templeman L, Roberts F. Effectiveness of expiratory muscle strength training on expiratory strength, pulmonary function and cough in the adult population: a systematic review. Physiotherapy. 2020;106:43-51. DOI:10.1016/j.physio.2019.06.002.
38. Cebria I Iranzo MA, Balasch-Bernat M, Tortosa-Chulia MA, Balasch-Parisi S. Effects of Resistance Training of Peripheral Muscles Versus Respiratory Muscles in Older Adults With Sarcopenia Who are Institutionalized: A Randomized Controlled Trial. J Aging Phys Act. 2018;26(4):637-46. DOI:10.1123/japa.2017-0268.
39. Liu K, Zhang W, Yang Y, et al. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract. 2020;39:101166. DOI:10.1016/j.ctcp.2020.101166.
About the Authors
E. D. BazdyrevRussian Federation
Evgeny D. Bazdyrev.
Kemerovo.
eLibrary SPIN 4545-0791
N. A. Terentyeva
Russian Federation
Natalia A. Terentyeva.
Kemerovo.
eLibrary SPIN 1323-2050
N. A. Galimova
Russian Federation
Natalia A. Galimova.
Kemerovo.
eLibrary SPIN 9296-4964
K. E. Krivoshapova
Russian Federation
Kristina E. Krivoshapova.
Kemerovo.
eLibrary SPIN 4272-7552
O. L. Barbarash
Russian Federation
Olga L. Barbarash.
Kemerovo.
eLibrary SPIN 5373-7620
Review
For citations:
Bazdyrev E.D., Terentyeva N.A., Galimova N.A., Krivoshapova K.E., Barbarash O.L. Respiratory Muscle Strength in Patients with Coronary Heart Disease and Different Musculoskeletal Disorders. Rational Pharmacotherapy in Cardiology. 2022;18(4):393-401. https://doi.org/10.20996/1819-6446-2022-08-04
















































