Scroll to:
Galectin-3 as a Marker of Cardiorenal Syndrome in Patients with Chronic Heart Failure
https://doi.org/10.20996/1819-6446-2022-04-04
Abstract
Aim. To assess the effect of renal dysfunction on the galectin-3 level in patients with chronic heart failure (HF) with preserved, intermediate and reduced left ventricular ejection fraction (EF).
Material and methods. Along with a clinical examination, 69 patients with HF (NYHA class II-IV) underwent tests for the level of NT-proBNP and galectin-3 in serum using enzyme immunoassay.
Results. Study participants were divided into 3 groups: 23 patients with preserved EF (HFpEF), 26 patients with midrange EF (HFmrEF), 20 patients with reduced EF (HFrEF). There was a trend to increase the concentration of galectin-3 with increase in NT-proBNP level. Correlation analysis showed significant feedback (r=−0.41, p<0.05) between galectin-3 and EF only in patients with preserved systolic function. In the same group of HFpEF patients, the maximum serum galectin-3 level was 10.5 [6.5; 14.5] ng/ml. Serum galectin-3 level showed negative correlated with the GFR in patients with CHF (r=−0.513, p<0.05). In patients with HF and glomerular filtration rate (GFR) <60 ml/min/1.73 m2 it was higher than in patients with GFR>60 ml/min/1.72 m2 (9 [5.3; 12.6] ng/mL vs 11.8 [6.2; 15.3] ng/mL, p<0.05). According to the ROC-analysis data, galectin-3 level >10.3 ng/ml indicates a high risk of chronic kidney disease stage 3-4 stage development (sensitivity 60%, specificity 75%) and can be considered as a risk factor for development of cardiorenal syndrome in HF patients.
Conclusion. Galectin-3 level in patients with HF is more influenced by the degree of reduction in GFR rather than the left ventricular systolic function impairment.
For citations:
Podzolkov V.I., Dragomiretskaya N.A., Kazadaeva A.V., Belyaev Yu.G., Tolmacheva A.V. Galectin-3 as a Marker of Cardiorenal Syndrome in Patients with Chronic Heart Failure. Rational Pharmacotherapy in Cardiology. 2022;18(2):153-159. https://doi.org/10.20996/1819-6446-2022-04-04
Introduction
Cardiorenal syndrome (CRS) is a special type of pathophysiological interaction between the heart and kidneys, in which acute or chronic dysfunction of one organ leads to acute or chronic dysfunction of another organ [1]. According to the accepted classification, renal dysfunction in patients with primary myocardial dysfunction, acute or chronic, is designated by the terms «acute kidney injury» and «chronic kidney disease», respectively [1]. Patients with chronic heart failure (CHF), as a rule, had type 2 CRS, characterized by the progression of chronic kidney disease against the background of chronic myocardial dysfunction [2].
The development of structural and functional disorders of internal organs, including the kidneys, is characteristic of stage III (dystrophic) according to the clinical classification of CHF from N.D. Strazhesko and V.Kh. Vasilenko [1]. Clearly, subclinical morphological and functional disorders of various internal organs are appearing at the earlier stages of CHF, which is caused by hypoperfusion of organs and tissues due to low ejection syndrome together with congestion. All this demands timely diagnosis of these preclinical processes for their correction and prevention of progression [3].
To date, the CRS pathogenesis is considered as a complex of hemodynamic and neurohumoral mechanisms leading to a progressive deterioration in the functional capacity of both organs [3-5]. The search for optimal biomarkers for early diagnosis of CRS is currently ongoing [2].
Researchers are interested in galectin-3, a galectin class protein expressed by various cells of the body (lymphocytes, macrophages, neutrophils, osteoclasts, fibroblasts), involved in stimulating cell growth, differentiation, activation of apoptosis, and playing an important role in inflammatory reactions, the development of fibrosis and myocardial remodeling, liver and kidneys [3][6-8].
We noted that a stable increase in galectin-3 in patients with CHF was associated with an increased risk of adverse outcome [9-11]. The results of the long-term DEAL-HF study (DEventer-ALkmaar Heart Failure study), which lasted more than 8 years, showed an inverse relationship between galectin-3 and glomerular filtration rate (GFR), regardless of the presence and severity of CHF [9], which allows us to consider galectin-3 not only as a cardiospecific marker of fibrosis, but also as a tool for assessing renal dysfunction in patients with CKD.
The aim of this research is to study the role of galectin-3 in the formation of CRS in patients with CHF with varying degrees of systolic dysfunction.
Material and methods
Characteristics of patients
The study included 69 patients (40 [ 58%] men and 29 [ 42%] women) with NYHA functional class II-IV CHF (FC), hospitalized at the University Clinical Hospital No. 4 (Sechenov University) with symptoms of CHF since November 2019 to March 2020. The diagnosis of CHF was confirmed using a comprehensive clinical and instrumental examination: anamnesis, physical examination data using the clinical assessment scale for CHF [1], echocardiography results and the NT-proBNP level > 125 ng/ml at admission. CHF was in all patients with a complication of coronary artery disease (CAD) and/or hypertension. All patients received standard CHF therapy according to clinical guidelines [1].
During the study, we followed the provisions of the Declaration of Helsinki and the principles of Good Clinical Practice. The study was approved by the Ethics Committee of the University. All patients signed an informed consent.
Exclusion criteria: malignant neoplasms, including those diagnosed during the present hospitalization; acute coronary syndrome, acute cerebrovascular accident during the previous 6 months; type 1 diabetes mellitus; chronic kidney disease (CKD) requiring hemodialysis; autoimmune or other inflammatory diseases (gastrointestinal tract, bronchopulmonary system, musculoskeletal system, etc.) in the acute phase.
Patients included in the study didn't meet the criteria for «acute decompensated heart failure» (ADHF) [1]. The symptoms didn't require hospitalization and treatment in the intensive care unit, and therefore the increase in creatinine level detected in them and the corresponding decrease in GFR was regarded as a manifestation of type 2 CRS, and not as acute kidney injure. Laboratory and clinical data of 55 patients (79.7%) who had anamnestic indications of previously diagnosed CKD also didn't meet the criteria for the diagnosis of acute kidney injure [1].
Examination methods
All patients underwent determination of the NTproBNP level using the enzyme immunoassay BI20852W Biomedica along with a standard general clinical examination - brain natriuretic propeptide (BNP-fragment, Austria), and blood serum galectin3 using enzyme-linked immunosorbent assay (Bender Med Systems, USA). Median values of the galectin3 level in groups of patients with different stages of CKD were calculated for a comparative assessment of the level of galectin-3 at different degrees of renal dysfunction [12]. Transthoracic echocardiography was performed according to the standard technique recommended by the American and European Society of Echocardiography using M- and B-modes using
the Toshiba Xario system (Japan), with the determination of the thickness of the interventricular septum and the posterior wall of the left ventricle, the mass index of the left ventricular myocardium, end systolic and diastolic sizes and volumes of the left ventricle, left ventricular ejection fraction (LV EF), systolic pressure in the pulmonary artery, etc. LV EF was determined by the Simpson method.
Patients included in the study were divided into 3 groups depending on the ejection fraction (EF) of the left ventricle (LV): with preserved (HFpEF; LV EF>50%; n=23), mid-range (HFmrEF; LV EF 40-50 %; n=26) and reduced LV EF (HFrEF; LV EF<40%; n=20). The stage of CKD was assessed based on the calculation of GFR using the CKD-EPI formula [10].
Statistical analysis
The results of the study were processed using the Statistica 10.0 program (Statsoft Inc., USA). The arithmetic mean (M) and standard deviation (σ) were calculated by statistical processing of data for variables with a normal distribution; median (Me) and interquartile range [25%;75%] were calculated for variables with non-normal distribution. Statistical significance of differences was assessed using Student's t-test (p [t] for normal distribution) and Mann-Whitney's test (p [U] for variables with non-normal distribution). Pearson's χ2 test was used when comparing frequency indicators to assess the significance of differences. Pearson's correlation coefficient (r for normal distribution) and Spearman's rank correlation coefficient (ρ for non-normal distribution) were used to identify and evaluate the relationships between the studied indicators. ROC-analysis with the definition of threshold values for the studied markers was carried out in order to assess the sensitivity and specificity of changes in the level of markers.
Results
The clinical characteristics of the patients included in the study are presented in Table 1.
Table 1. Clinical characteristics of the examined groups
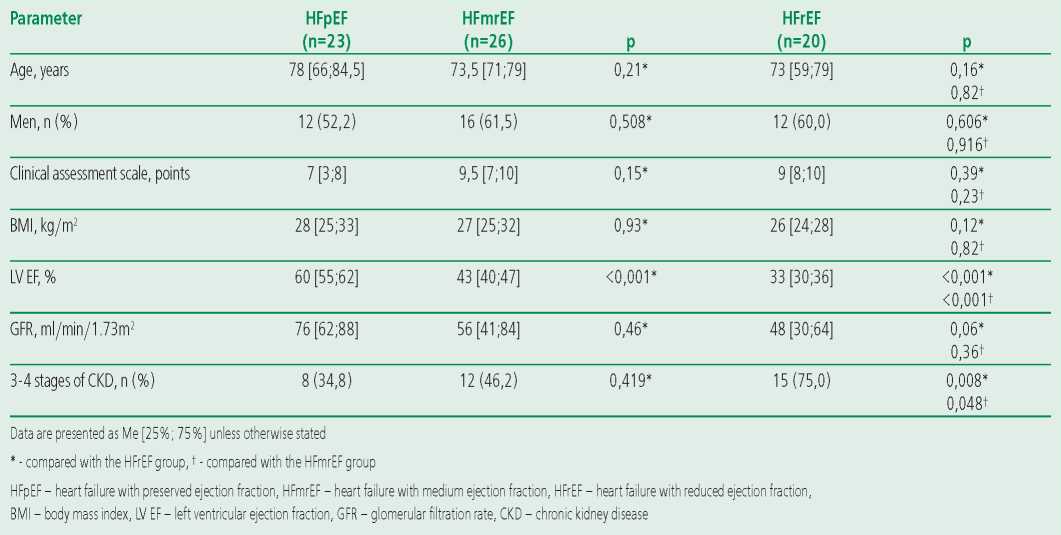
All patients had clinical manifestations of severe CHF, corresponding mainly to FC III-IV (7-9.5 points according to the clinical assessment scale). At the same time, the groups of patients with HFpEF, HFmrEF and HFrEF were comparable in terms of the studied clinical and demographic parameters, but high levels of GFR were noted significantly more in the HFpEF group when compared with the HFmrEF and HFrEF groups.
Median values of the galectin-3 level in groups of patients with different stages of CKD were calculated for its comparative assessment at different severity of renal dysfunction (Fig. 1): CKDc2 (GFR≥60ml/min/1.73m2; n=35), CKDc3a (GFR=45-59 ml/min/1.73m2; n=17), CKDc3b (GFR=30-44 ml/min/1.73m2; n=14) and CKDc4 (GFR=15-29 ml/min/1.73m2; n=5). We found a significant increase in the concentration of galectin3 in the blood serum with a decrease in GFR up to CKDc3b (Fig. 2) in the absence of significant differences in the galectin-3 level in patients with varying degrees of left ventricular systolic dysfunction (Table 2).
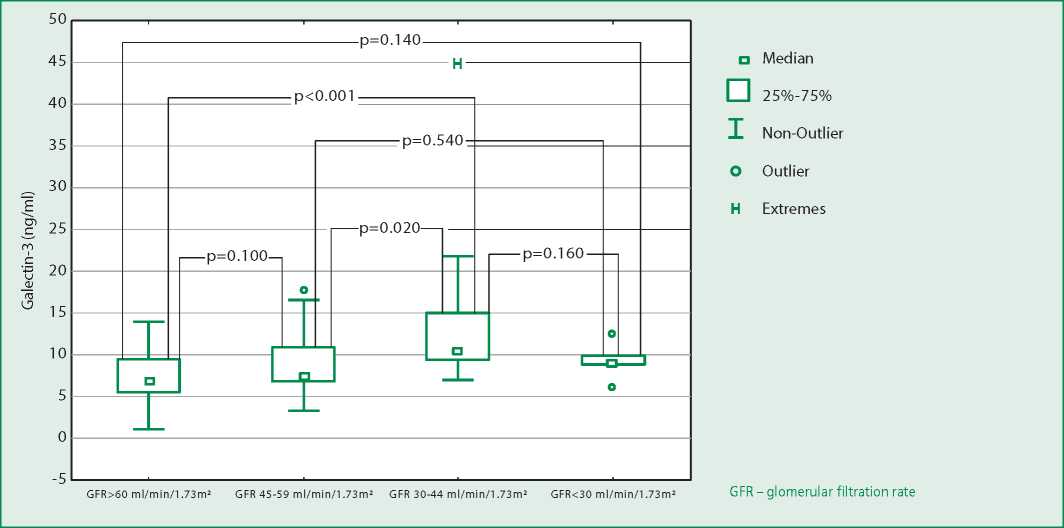
Figure 1. Galectin-3 levels in chronic heart failure patients with different stages of chronic kidney disease
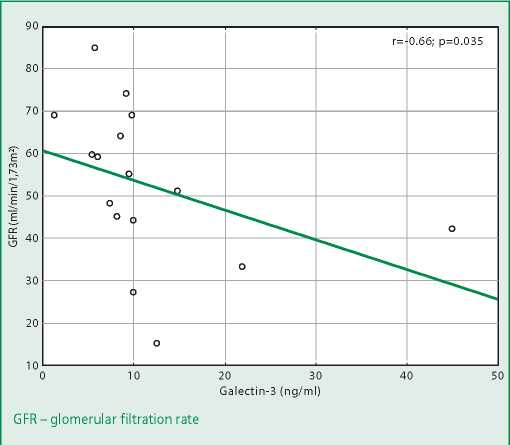
Figure 2. Correlation of galectin-3 level and glomerular filtration rate value in patients with heart failure with reduced ejection fraction
Table 2. Indicators of left ventricular ejection fraction in patients with different stages of chronic kidney disease
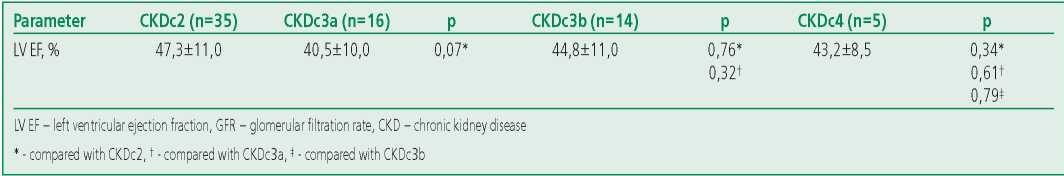
A significant negative correlation between the galectin-3 level and GFR was found both for all patients with CHF and in the subgroups of HFrEF and HFpEF, but not HFmrEF (Table 3).
Table 3. Correlation between the level of galectin-3 and glomerular filtration rate in patients with chronic heart failure with varying degrees of left ventricular systolic dysfunction

ROC analysis was performed to assess the threshold values of galectin-3 that increase the risk of having stage 3-4 CKD, as well as to calculate the sensitivity and specificity of this marker. When analyzing the predictive value of galectin-3 and the ROC curve, we found that the galectin-3 level >10.3 ng/ml with a sensitivity of 60% and a specificity of 75% increases the risk of a decrease in GFR<60 ml/min/1.73m2 in patients with CHF. AUC=0.753 (St 0.057) (Fig. 3).
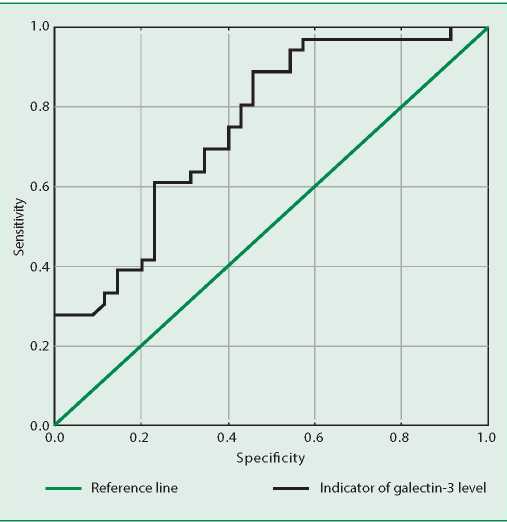
Figure 3. ROC analysis for the absolute value of galectin-3 associated with an increased risk of glomerular filtration rate reduction <60 ml/min/1.73 m2 in patients with chronic heart failure
Discussion
To date, the physiological and pathophysiological effects of galectin-3 have been well studied and described, including in patients with CHF. But the inconsistency of the results of randomized clinical studies, such as CARE-HF (Cardiac Resynchronization in Heart Failure), CORONA (Controlled Rosuvastatin MultiNAtional Trial in Heart Failure) [6][11], as well as smaller studies [12-18] aimed at studying diagnostic and prognostic value of galectin-3, still doesn't allow to reliably determine the indications for the use of this marker in clinical practice [19]. Also, the reference values of galectin-3 for healthy individuals have not yet been determined, and there are no unambiguous data to judge the degree of its increase in patients with CHF.
The lack of consensus among scientists regarding the threshold values of galectin-3 tells us about the need for further studies in this direction. In our work, the values of galectin-3 were somewhat lower compared to the results of other authors [12-15][18], but significantly higher values were noted in patients with stage 3-4 CKD, which is consistent with the results of previous studies and confirms representativeness of our group of patients with CHF and the significance of the results obtained.
It's widely known that hypertension and coronary artery disease as the main causes of CHF make a significant contribution to the development of CKD. Diffuse hemodynamic disorders mediated by hyalinosis and sclerosis of the coronary and renal arteries, as well as humoral regulatory factors, including many biologically active substances, including galectin-3, lead to the formation of cardiorenal syndrome and progression of the cardio-renal continuum [9][11][13][20]. At the same time, most researchers explain the relationship between elevated levels of galectin3 and renal dysfunction precisely by the development of renal fibrosis in patients with CHF, and not by a decrease in the renal clearance of galectin-3 [3][12][17][20].
In our study, we drew attention to an upward trend in galectin-3 levels in patients with higher stages of CKD, traceable up to CKDc3b (Fig. 1). Patients with CKDc4 observed a slight decrease in the galectin-3 level compared with CKDc3b and CKDc3a, which may be due to the small sample size (n=5).
The finding of a significant negative correlation between galectin-3 levels and GFR in patients with HFrEF and HFpEF is consistent with similar data from earlier animal studies and some studies and metaanalyses that showed a negative correlation between galectin-3 expression and serum creatinine levels in patients with kidney disease [21][22]. This fact confirms the important role of galectin-3 in the process of fibrosis, which accompanies the early stages of CKD formation. But we didn't find threshold values for the galectin-3 level in patients with CHF and CKD in the literature available to us.
According to the results of the ROC analysis performed in our study, the galectin-3 level of >10.3 ng/ml with a sensitivity of 60% and a specificity of 75% indicates a high risk of stage 3-4 CKD and can be considered as an indicator of the development of cardiorenal syndrome in patients with CHF.
The obtained results reflect the diagnostic value of this biomarker of fibrosis in patients with CHF, but the fact that galectin-3 is a marker of fibrosis not only of the myocardium, but also of other organs, primarily the kidneys, requires an assessment of comorbid conditions [23]. And the determination of the galectin-3 level in the blood serum of patients with CHF allows us to indirectly clarify the degree of activity of fibrotic processes both in the myocardium, including in the early stages of the formation of the disease, with still preserved LV EF, and in the renal parenchyma, as well as to assess the risk development of CKD in them, which is an additional factor of poor prognosis in CHF [1].
According to the results of the observations of F.J. Carrasco-Sanchez et al, predicting poor prognosis in patients with acute heart failure and preserved EF along with age, NYHA FC, anemia and diabetes mellitus, elevated brain natriuretic peptide levels, decreased GFR and hyponatremia, was an increase in the galectin-3 level >13 .8 ng/ml (odds ratio was 1.43, 95% confidence interval was 1.07-1.91; p=0.015) [24]. And in the CARE-HF study, plasma galectin-3 levels >30 ng/ml were found to be prognostically unfavorable and associated with a high risk of endpoints (death and hospitalization due to CHF) in patients with CHF (NYHA class III-IV) with signs LV systolic dysfunction [6].
Galectin-3 values reached such high values only in two of our patients. These were elderly women with an ischemic nature of CHF with clinical and radiographic signs of pneumonia, with a decrease in GFR<45 ml/min/1.73 m2. The maximum value of galectin-3 (60 ng/ml) was observed in a 69-yearold patient with an LV EF of 34% and clinical manifestations of severe «congestive» stage III CHF (in the form of anasarca, ascites and bilateral hydrothorax), NYHA IV FC, which developed as a result of myocardial infarction and permanent form of atrial fibrillation against the background of long-term poorly controlled hypertension; the patient also suffered from grade 1 obesity and mild anemia, she had a decrease in GFR (CKD-EPI) to 33 ml/min/1.73 m2, consistent with CKDc3b.
Conclusion
Our study showed that an increase in the concentration of serum galectin-3 in patients with CHF is largely determined not by a violation of the left ventricle systolic function, but by a decrease in the glomerular filtration rate. At the same time, the achievement of a threshold value of serum galetin-3 of 10.3 ng/ml can be considered as an indicator of the development of cardiorenal syndrome in patients with CHF, which significantly worsens the further course of the disease and increases the risk of adverse outcomes.
Relationships and Activities: none.
Funding: The study was performed with the support of the Sechenov University.
References
1. Mareev VYu, Fomin IV, Ageev FT, et al. Russian Heart Failure Society, Russian Society of Cardiology. Russian Scientific Medical Society of Internal Medicine Guidelines for Heart failure: chronic (CHF) and acute decompensated (ADHF). Diagnosis, prevention and treatment. Kardiologiia. 2018;58(6S):8-158(In Russ.) DOI:10.18087/cardio.2475
2. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527-39. DOI:10.1016/j.jacc.2008.07.051.
3. Frangogiannis N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70-99. DOI:10.1016/j.mam.2018.07.001.
4. Suthahar N, Meijers WC, Silljé HHW, et al. From inflammation to fibrosis-molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep. 2017;14(4):235-50. DOI:10.1007/s11897-017-0343-y.
5. Ibrahim N, Januzzi JL. The potential role of natriuretic peptides and other biomarkers in heart failure diagnosis, prognosis and management. Expert Rev Cardiovasc Ther. 2015;13(9):1017-30. DOI:10.1586/14779072.2015.1071664.
6. Lopez-Andrès N, Rossignol p, Iraqi W, et al. Association of galectin-3 and fibrosis markers with longterm cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur J Heart Fail. 2012;14(1):74-81. DOI:10.1093/eurjhf/hfr151.
7. Gao Z, Liu Z, Wang R, et al. Galectin-3 is a potential mediator for atherosclerosis. Immunol Res. 2020;2020:5284728. DOI:10.1155/2020/5284728.
8. Amin HZ, Amin LZ, Wijaya IP, Galectin-3: a novel biomarker for the prognosis of heart failure. Clujul Med. 2017;90(2):129-32. DOI:10.15386/cjmed-751.
9. Lok DJ, Van Der Meer P, de la Porte PW, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study Clin Res Cardiol. 2010; 99(5):323-8. DOI:10.1007/s00392-010-0125-y.
10. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4S):S1-S276. DOI:10.1016/j.kint.2021.05.021.
11. Gullestad L, Ueland T, Kjekshus J, et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur Heart J. 2012;33(18):2290-96. DOI:10.1093/eurheartj/ehs077.
12. Dubolazova YuV, Drapkina OM. Galectin-3 and NT-proBNp as biomarkers of heart failure decompensation. Russ J Cardiol. 2017;141(1):95-101 (In Russ.) DOI:10.15829/1560-4071-2017-1-95-101.
13. Karetnikova VN, Osokina AV, Evseeva MV. Relationship between blood serum galectin and renal dysfunction in ST elevation myocardial infarction. Kardiologiia. 2016;56(4):25-31 (In Russ.) (In Russ.) DOI:10.18565/cardio.2016.4.25-31.
14. Kurbonov AK, Gadaev AG, Nurillaeva NM, et al. Galectin-3: role in the formation of various hemodynamic phenotypes of heart failure and interaction with some neurohumoral factors. Russ J Cardiol. 2020;25(7):3476 (In Russ.) DOI:10.15829/1560-4071-2020-3476.
15. Zolotovskaya IA, Davydkin IL, Duplyakov DV, Kokorin VA. Cardiorenal relationships in the focus of risks of atrial fibrillation in patients after acute ST-elevated myocardial infarction (observational program FAKEL). Rational pharmacotherapy in Cardiology 2019;15(2):159-65 (In Russ.) DOI:10.20996/1819-6446-2019-15-2-159-165
16. Binas D, Daniel H, Richter A, et al. The prognostic value of sST2 and galectin-3 considering different aetiologies in non-ischaemic heart failure. Open Heart. 2018;5(1):e000750. DOI:10.1136/openhrt2017-000750.
17. Koukoui F, Desmoulin F, Galinier M, et al. The prognostic value of plasma galectin-3 in chronic heart failure patients is maintained when treated with mineralocorticoid receptor antagonists. PLoS One. 2015;10(3):e0119160. DOI:10.1371/journal.pone.0119160.
18. Schmitt VH, Prochaska JH, Föll AS, et al. Galectin-3 for prediction of cardiac function compared to NT-proBNP in individuals with prediabetes and type 2 diabetes mellitus. Sci Rep. 2021;11(1):19012. DOI:10.1038/s41598-021-98227-x.
19. Ponikowski P, Voors AA, Anker SD, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-200. DOI:10.1093/eurheartj/ehw128.
20. Gopal DM, Kommineni M, Ayalon N, et al. Relationship of plasma galectin-3 to renal function in patients with heart failure: effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J Am Heart Assoc. 2012;1(5):e000760. DOI:10.1161/JAHA.112.000760.
21. Rebholz CM, Selvin E, Liang M, Ballantyne CM. Plasma galectin-3 levels are associated with the risk of incident chronic kidney disease. Kidney Int. 2018;93(1):252-9. DOI:10.1016/j.kint.2017.06.028.
22. Zhang T, Cao S, Yang H, Li J. Prognostic impact of galectin-3 in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol. 2019;51(6):1005-1011. DOI:10.1007/s11255-019-02123-3.
23. Kim AJ, Ro H, Kim H, et al. Soluble ST2 and Galectin-3 as predictors of chronic kidney disease progression and outcomes. Am J Nephrol. 2021;52(2):119-30. DOI:10.1159/000513663.
24. Carrasco-Sanchez FJ, Aramburu-Bodas O, Salamanca-Bautista P, et al. Predictive value of serum galectin-3 levels in patients with acute heart failure with preserved ejection fraction. Int J Cardiol. 2013:169(3):177-82. DOI:10.1016/j.ijcard.2013.08.081.
About the Authors
V. I. PodzolkovRussian Federation
Valery I. Podzolkov
Moscow
N. A. Dragomiretskaya
Russian Federation
Natalia A. Dragomiretskaya
Moscow
A. V. Kazadaeva
Russian Federation
Anna V. Kazadaeva
Moscow
Yu. G. Belyaev
Russian Federation
Yury G. Belyaev
Moscow
A. V. Tolmacheva
Russian Federation
Anastasia V. Tolmacheva
Moscow
Review
For citations:
Podzolkov V.I., Dragomiretskaya N.A., Kazadaeva A.V., Belyaev Yu.G., Tolmacheva A.V. Galectin-3 as a Marker of Cardiorenal Syndrome in Patients with Chronic Heart Failure. Rational Pharmacotherapy in Cardiology. 2022;18(2):153-159. https://doi.org/10.20996/1819-6446-2022-04-04
JATS XML
















































